 |
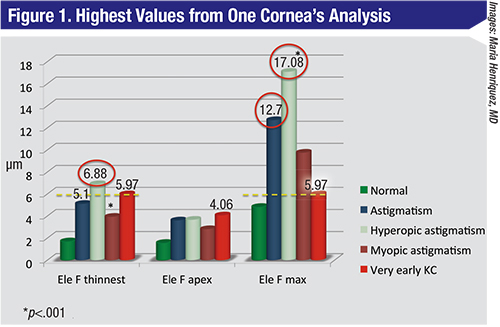
| When the front surface elevation values of a high-ammetropic cornea are analyzed, astigmatism and hyperopic astigmatism stand out more than very early keratoconus. |
Lima, Peru, ophthalmologist Maria Henriquez says that detecting an asymmetry between a patient’s eyes, rather than just focusing on one eye, might help catch keratoconus preop.
“Keratoconus is a bilateral disease,” Dr. Henriquez explains. “However, unlike glaucoma, which usually gets a bilateral evaluation, keratoconus doesn’t get a bilateral analysis. So, we performed a study to analyze the tomography parameters in eyes with keratoconus in order to develop a bilateral asymmetry index.”
In a prospective study presented at the 2015 meeting of the American Society of Cataract and Refractive Surgery, Dr. Henriquez and her co-workers used the Pentacam to analyze 424 patients (748 eyes). Of these patients, 294 had bilateral keratoconus, 50 had high myopia or astigmatism, 30 had forme fruste keratoconus, and 50 had low ammetropia and were used as a control group. The researchers studied 30 parameters, including central corneal thickness, pachymetry at the thinnest point and posterior elevation at the thinnest point. Among other results, they found that the mean asphericity asymmetry in the keratoconus group was 0.36 µm compared with 0.05 in the high ammetropic group (p<0.001). The mean steep keratometry asymmetry in keratoconus was 2.26 D compared with 0.48 D in the high-ammetropic group (p=0.01), and the mean anterior elevation asymmetry at the corneal apex was 7.34 µm compared with 1.61 µm in the high-ammetropic group (p=0.03). Asphericity had an area under the receiver operating characteristic curve of 0.97 in discriminating between keratoconus and high-ammetropic eyes.
Dr. Henriquez says the results showed there’s a greater asymmetry in eyes with keratoconus than in normals or eyes with high refractive errors. “With the model we developed,” she explains, “if we compare very early keratoconus—defined as both eyes having K values less than 48 D—with normal eyes, we’ve found a sensitivity and specificity of 0.92. However, when we add a non-asymmetric variable called the Final D, a percentage derived from logistic regression of all the parameters, sensitivity and specificity increases to 0.98.”
There are certain patient presentations that can confound the asymmetry index. “If a patient has had previous surgery in one eye but not the other, you’re going to find a larger asymmetry value, but it’s not keratoconus.
“We’ve calculated the normal asymmetry value in the normal population,” Dr. Henriquez continues. “This value plus two standard deviations is the normal range. If the value is outside this, the risk is increased. This is similar to OCT glaucoma detection systems.”
The asymmetry index isn’t currently on a device, but Dr. Henriquez says she and her colleagues are working with Oculus at the moment, though no agreement has been finalized. If a surgeon doesn’t have access to a Pentacam, or doesn’t want to wait until the index comes to that device, he can do the asymmetry calculation, though it will be more involved. “You have to look at the parameters I suggest in our paper in order to calculate the asymmetry, and you can get the normal value for comparison because it’s published in our paper.1 You can compare the asymmetry value you get with the normal value. It will be more difficult to do, but you can do it.”
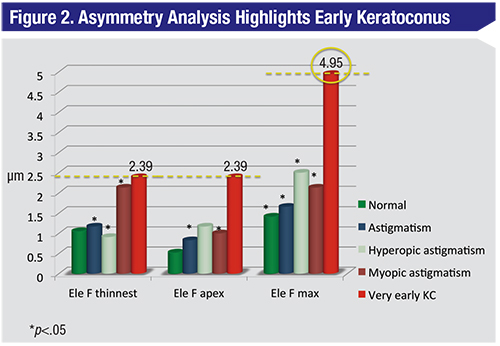 |
| When a bilateral evaluation of the cornea from Figure 1 and its fellow is performed, suddenly the very early keratoconus stands out as the highest value. |
Marcony Santhiago, MD, PhD, of Rio de Janeiro, Brazil, acknowledges that parameters such as the residual corneal bed post-LASIK and the flap thickness play roles in ectasia risk, but thinks they’re just part of the equation. More important, he argues, is the percentage of the tissue that’s altered by the entire procedure.
Dr. Santhiago says PTA is represented by the following equation: PTA = (FT+AD)/CCT. In the equation, FT is flap thickness, AD is ablation depth and CCT is the preoperative central corneal thickness. “This concept, in my opinion, better describes the interaction between flap thickness, ablation depth and corneal thickness,” Dr. Santhiago says. “Our studies show that, in eyes with normal preop topography, PTA has a higher prevalence, presents the higher odds ratio and a higher predictive capability for ectasia risk than the values of residual stromal bed, central corneal thickness, ablation depth or age.2 In our studies, the risk of ectasia rapidly increases with a PTA higher than 35 percent—with 100-percent sensitivity—and its combination of sensitivity and specificity peak when PTA is equal to or higher than 40 percent.” He adds that, in eyes with abnormal topography, less tissue alteration is necessary to induce ectasia. “Even subtle signs of abnormal topography are associated with ectasia after minimal tissue removal, and therefore there is no safe limit in such a situation.”
Dr. Santhiago says PTA may be more than the sum of its parts, since it combines the effects of the flap and the ablation. “There is a common thought that the flap thickness is the only factor responsible for ectasia, or that ectasia only occurs in eyes that have the so-called, ‘thicker-than-expected’ flaps,” he says. “We conducted a study to specifically address this matter. We investigated groups perfectly matched for PTA and flap thickness: In the first group were eyes with the same PTA, some of which developed ectasia and some of which didn’t; and in the second group were eyes with the same flap thickness, some of which developed ectasia and some of which didn’t. In the first group, we did find that the group that developed ectasia had thicker flaps. In the second group, the patients who developed ectasia had a greater ablation depth. Therefore, we found that the LASIK flap had a greater impact than ablation depth, but that thicker flaps alone were insufficient to create ectasia unless coupled with greater ablation depths—meaning high PTA values. As a result, it appears that PTA presents higher discriminative capabilities than each of the variables that comprise it.”
Dr. Santhiago says the same goes for the metrics of residual bed thickness and central corneal thickness, which many surgeons initially used as guideposts for screening. “Residual bed thickness theoretically informs you about the remaining load-bearing tissue after surgery, but 250 or 300 µm would mean different biomechanical changes depending on how much tissue you’ve altered to reach that value,” he explains. “This concept was proposed assuming that the cornea had a homogeneous distribution of tensile strength, which isn’t true. Since corneal tensile strength is not uniform throughout the central cornea, but instead has a progressive weakening in the posterior two-thirds, it seems reasonable that a ratio or a percentage wouldn’t only be more representative of the actual change that has occurred, it’s also a more individualized metric. This concept is the basis for PTA.”
The Santhiago PTA display is a feature available on the Ziemer Galilei, which isn’t approved by the Food and Drug Administration. However, it’s conceivable that a surgeon with a reliable pachymeter could do the calculation himself to get a better idea of a patient’s risk for developing ectasia. “As a risk factor, the weakening predicted by a high PTA, or any other factor, doesn’t mean that ectasia will occur in all high-risk eyes,” Dr. Santhiago notes. “It merely means these eyes carry an increased risk. It seems logical, though, that the balance of risk should be weighted toward minimizing it, especially when other excellent procedures are available. If PTA is the only risk factor, the surgeon should change to surface ablation and the patient would fall within safe limits.” REVIEW
Dr. Santhiago is a consultant to Ziemer. Dr. Henriquez has no financial interest in the products discussed.
1. Henriquez MA, Izquierdo L, Belin MW. Intereye asymmetry in eyes with keratoconus and high ammetropia: Scheimpflug imaging analysis. Cornea 2015;34:10:S57–S60.
2. Santhiago MR, Smadja D, Gomes BF, et al. Association between the percent tissue altered and post-laser in situ keratomileusis ectasia in eyes with normal preoperative topography. Am J Ophthalmol 2014;158:1:87-95.