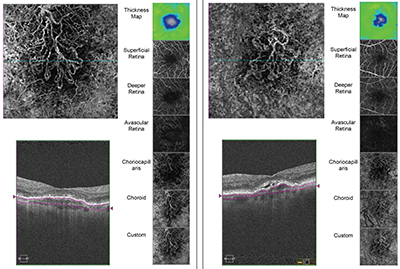
 |
| OCT angiography reveals movement within blood vessels over short periods of time, allowing surgeons to follow the impact of a disease on the retinal microvasculature. The ability to use custom segmentation to examine specific layers of the retina can be crucial. Above: scans of two eyes with choroidal neovascularization. (Image courtesy Philip Rosenfeld, MD, PhD.) |
Scanning the Microvasculature
Thanks to a recent Food and Drug Administration approval, Zeiss’s AngioPlex OCT system, which can perform OCT angiography, is now becoming available to surgeons in the United States. Philip Rosenfeld, MD, PhD, professor of ophthalmology at the Bascom Palmer Eye Institute at the University of Miami Miller School of Medicine, has worked with Zeiss for several years helping to develop and refine the instrument. “OCT angiography is going to revolutionize the way we manage patients in clinic,” he says. “Of course, some retina specialists are skeptical, as many of us were when earlier versions of OCT were introduced. Doctors want to know what OCT angiography will reveal that they couldn’t elucidate from routine spectral-domain OCT imaging. The answer is that OCT angiography allows us to follow pathology better than we’ve ever been able to before. Now we can not only image structure, we can follow anything that moves through a blood vessel, and hence anything that comprises the microvasculature of the retina and the choroid, over time. That’s really important because many of the diseases we treat are diseases of the microvasculature.”
A technology like OCT angiography will clearly have value as a research tool, but Dr. Rosenfeld says it’s also helping him in the clinic. “It has already affected our patient management in at least three areas,” he says. “First, it’s helping us with early detection of new blood vessels in macular degeneration and early recurrence of blood vessels in wet macular degeneration patients undergoing treatment. Second, it’s helping me to exclude a diagnosis of neovascularization in patients who have what I call masquerade conditions. These patients appear to have wet macular degeneration or active neovascularization that’s leaking, but actually have central serous retinopathy, uveitis or vitelliform lesions, or what we call dysfunctional RPE syndrome, where there’s fluid under the retina but it’s not a VEGF-mediated process. This technology gives us a better idea of who really has neovascularization and needs to be treated.
“It’s also very useful for following diabetic patients with neovascularization,” he notes. “For the first time we can actually quantify the neovascularization in the macula and see if it’s changing. In the past, we would look at the macula and get a fluorescein angiogram. The leakage would make it difficult to see the entire lesion, and of course, we wouldn’t repeat the fluorescein angiogram at every visit. But we can repeat OCT angiography very easily and follow the location of the neovascularization, its size, its thickness, its elevation and how the neovascularization is changing, down to the micron. This really is having a clinical impact; it often causes us to change our treatment. It gives us a concrete, unambiguous way to follow these neovascular lesions.”
How It Works
“What’s really nice about the Zeiss AngioPlex OCT instrument is that you still have all of the imaging capabilities that you would have with standard spectral-domain OCT—all the B-scans, the raster scans, the volumetric data sets, the thickness maps and the en face imaging,” he says. “Essentially, it’s a spectral-domain instrument with an added advantage. So when you do the scan pattern for OCT angiography, you can look at the data in two ways: You can look at it in the structural format, in which you get a routine B-scan and dataset that you can segment, measure or look at the en face structural images; and you can also get en face flow images.
“Each OCT angiographic instrument uses different algorithms to generate the flow image, so I’ll just discuss the AngioPlex device, which I’m most familiar with,” he continues. “To perform angiography, the instrument rapidly takes multiple B-scans at the same position. If you scan a 3 x 3-mm area, it takes four B-scans in one position; then it moves to the next position about 12 µm away and does another four B-scans in that position. In total, it takes just over four seconds to get the scans, and you can accomplish this through an undilated pupil. You can also scan a 6 x 6-mm area; in that mode the instrument repeats each B-scan twice instead of four times. Because the 3 x 3 scan has a higher density of B-scans and more repetition, the image quality is better, showing more detail. Generally, we image a large area first; then we hone in on smaller areas of interest and get higher-quality flow maps of those areas. Of course, you can create a montage to map out a larger area, but most of the time a 6 x 6-mm scan will give you most of what you need.”
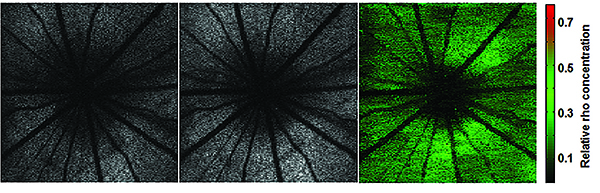 |
| OCT done with visible light can be used to quantify rhodopsin, a way to gauge the health of photoreceptors. Above: A rat retina was imaged in the dark-adapted stage (left panel) and then in the light-adapted stage (middle panel). Rhodopsin distribution was calculated by the differential image of the two (right panel). The images were processed with a novel speckle-redistribution algorithm. (Image courtesy Shuliang Jiao, PhD.) |
Dr. Rosenfeld adds that one of the nicest features of the AngioPlex system is that it’s just a software and hardware upgrade if you already use the Cirrus OCT 5000. “Obviously an upgrade is less expensive than buying a whole new instrument,” he says. “Technicians just need to manipulate the hardware and software, which can be done with a service call.”
Limitations
Dr. Rosenfeld notes that current OCT angiography technology has limitations, but argues that they have little impact on its usefulness. “Some surgeons have pointed out that you can’t see leakage with OCT angiography,” he says. “That’s true; you don’t see fluorescein leaking out of vessels. But leakage can be detected indirectly by just looking at macular edema—the presence of increased retinal thickness. I can only think of one disease where leakage doesn’t cause increased thickening of the retina: that’s macular telangiectasia type II. Luckily, this disease is easily diagnosed with OCT structural images and OCT angiography, so that’s not much of a disadvantage.
“People also argue that ICG angiography gives you a better image of the choroid than spectral-domain OCT angiography,” he continues. “That’s probably true, because ICG angiography may provide better penetration into the choroid. But spectral-domain technology is pretty good; you see the choriocapillaris; you see the choroid; and in our experience we’ve been able to see everything using spectral-domain OCT angiography that we were able to see using ICG angiography. So I don’t think you’re sacrificing anything.”
Dr. Rosenfeld says that artifacts may still appear in a scan, but says the companies are working to correct that. “When you look at deep retinal structures, anything the light passes through on the way to those structures that contains blood vessels will create what’s called a projection artifact,” he says. “The major retinal blood vessels will look like they’re appearing deep in the retina and choroid. The images are faint, but they’re there. Right now we don’t have ways to get rid of those artifacts, but Zeiss is developing an algorithm that will remove them from the images.”
Other than that, Dr. Rosenfeld says the current image quality is excellent. “The company has improved the eye-tracking system, which is crucial when you’re repeating scans,” he notes. “After all, the only thing that should change between scans is the light scattering from the flow of blood cells through the microvasculature. That requires correcting for eye movement with micron-level precision.
“This technology is only going to get better and faster,” he adds. “You’ll be able to scan larger areas; and the future probably belongs to swept-source OCT where you have better penetration through the RPE for better choroidal imaging.”
Monitoring Photoreceptor Health
Another new use of OCT—measuring rhodopsin in the retina—is under development in a joint effort by researchers at Bascom Palmer Eye Institute and Florida International University in Miami. Because rhodopsin plays a key role in the function of rod photoreceptors, measuring it is a promising way to judge the condition of photoreceptor health, as well as the impact of treatments intended to preserve vision.1 What separates this approach from previous methods of rhodopsin measurement is that OCT technology allows the location of the rhodopsin to be determined with precision in vivo, including the layer of the retina it is in. Because it can measure within a specific layer, this approach eliminates artifacts that limit the value of other measurement methods. And unlike standard OCT, this technology uses visible light (hence the name VIS-OCT). Here, three of the researchers developing this technology discuss their work.
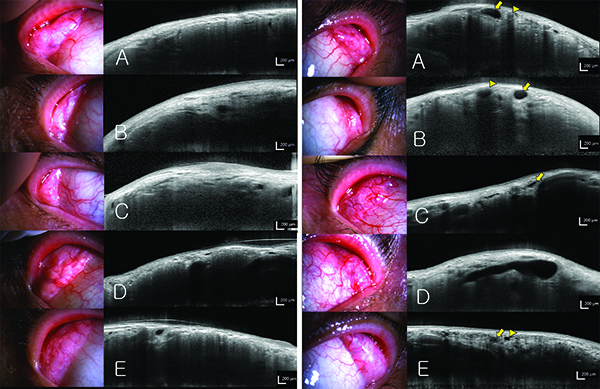 |
| OCT can be used to image the lacrimal glands, potentially helping to identify and treat dry eye. Above left: OCT scanning of exposed lacrimal glands. In 88 percent of subjects the parenchyma was clearly visible (A,B). In the other 12 percent of subjects, the margin of the lacrimal gland was indefinite and the parenchyma was not clearly visible (C). The acinar structures could be observed, as long as the lacrimal gland was well-exposed, the conjunctiva and subconjunctival tissue were thin and eyeball movement during the scan was minimal (D,E). Above right: The excretory ducts of lacrimal glands. Blood vessel lumens (arrow head) have higher signal intensity (grey), while excretory duct lumens (arrow) have minimal signal intensity (black). Cross-sections of blood vessels usually appear circular, but cross-sections of the excretory ducts usually appear ellipsoid or distorted ellipsoid (A). Behind the blood vessels (arrow head), a dark shadow appears, like an acoustic shadow in ultrasonography. Behind the excretory duct (arrow), a ‘negative shadow’ (higher signal intensity) is observed (B). Compared to blood vessels, the excretory duct wall is thick and has a high signal intensity (C). Among patients with dry-eye syndrome, the excretory ducts were excessively dilated, possibly because of obstruction of the excretory duct opening (D). The lumens of some ducts (arrow head) have minimal signal intensity like excretory ducts (arrow), but the walls are very thin; these ducts were considered to be dilated interlobular ducts or lymphatic vessels, rather than excretory ducts (E). (Images courtesy Hosik Hwang, MD, PhD.) |
Rong Wen, MD, PhD, professor of ophthalmology at Bascom Palmer Eye Institute, says the image processing is similar to standard OCT, but the procedure and the algorithms are different. “In our current protocol, we image the retina twice,” he says. “To measure rhodopsin you want to first image the dark-adapted rhodopsin, which absorbs the wavelengths of the probing light, around 520 µm. Then we remeasure after the rhodopsin has been light-adapted. The difference between the two reveals the amount of absorption done by the rhodopsin. By calculation you can figure out how much rhodopsin is in the retina at any given spot.
“This idea is not new,” he continues. “However, this OCT approach is new because it provides depth resolution, which means you can see how different layers contribute to the absorption. That allows us to remove layers that are not relevant to the photoreceptors to generate a better measurement. Also, this method allows us to measure the distribution of rhodopsin, which is a new benefit.”
Dr. Jiao notes that the use of visible light doesn’t bother the animals currently being tested. “The light is visible, but it’s pretty weak,” he says. “The light power in the current system is less than 240 microwatts, which is very similar to current commercial machines used for lipofuscin autofluorescence imaging. So although it’s visible, it’s tolerable. We don’t foresee any problem when this is used in human eyes.”
So far, the researchers have demonstrated that the system is measuring only rhodopsin, but they haven’t had the funding to compare the quantitative VIS-OCT measurement to other types of rhodopsin measurements. “There will always be limitations to the accuracy of any measurement,” notes Byron L. Lam, MD, the Robert Z. and Nancy J. Greene Chair in Ophthalmology at Bascom Palmer. “We don’t yet know exactly how precise this measurement is. Also, we’ve only imaged in animals so far, so we may have to adjust the technology’s field of view for human eyes. We hope to image human eyes soon.”
Dr. Wen notes that rat eyes and human eyes use rhodopsin in similar ways. “There’s no reason this technology cannot apply to humans,” he says. “There will just be some technical issues because the size of the eyes is different.”
Dr. Lam sees this technology being useful both for research and in the clinic. “Researchers will be able to recreate retinal diseases in the laboratory and test different therapeutic strategies,” he says. “It will also be very useful in future clinical trials of new treatments. The other important use will be in the clinic, where I see two different scenarios. First of all, you can use it in the clinic to follow a patient, to determine whether a retinal disease has progressed. You won’t have to rely on the typical subjective tests that we do today, such as visual field and visual acuity. And if you’re treating the patient, this will help you determine whether the treatment is working or not.”
Dr. Lam says they hope that this sort of functional, objective measure will open the door for other types of functional OCT testing in the future. “The ultimate goal would be to have a functional, objective way of looking at not just photoreceptor cells, but other cells that have other functions. I think that day will come. This is just the first step.”
A New Look at Dry Eye
A third new use for OCT—imaging the lacrimal glands to help identify dry-eye syndrome—is being studied at a group of universities and hospitals in South Korea. Although several instruments are now available for evaluating the meibomian glands, few are designed to provide information about the tear-producing lacrimal glands. Although the work is still in its early stages, these researchers have shown that anterior segment OCT has the potential to reveal the health of these glands in ways previously not possible.
“The lacrimal glands are the most important glands in dry-eye syndrome, but there have been few techniques for imaging them,” says Hosik Hwang, MD, PhD, in the department of ophthalmology at Chuncheon Sacred Heart Hospital, Hallym University, in Chuncheon, Korea. “I realized that OCT scanning of the exposed palpebral lobe could be used to visualize the parenchyma of the lobe just beneath the conjunctiva. This type of imaging could be used in Sjögren’s syndrome, graft-versus-host disease and other diseases that involve the lacrimal glands.”
Dr. Hwang’s team conducted a study in which anterior segment OCT was used to obtain cross-sectional images of subjects’ lacrimal gland palpebral lobes, in vivo.2 “The examiner pulled the temporal part of the upper eyelid in the superotemporal direction without everting it and asked the subject to look in the inferonasal direction,” he says. “Then we obtained B-scans longitudinally or transversely relative to the exposed palpebral lobe. The images allowed us to identify the excretory ducts, lobules, inter- and intralobular ducts, parenchyma and acini of the palpebral lobe.” (See examples, facing page.)
Dr. Hwang points out that alternatives for imaging these glands are not practical in dry-eye patients. “OCT scanning can visualize the tomogram of lacrimal glands non-invasively,” he says. “If the lacrimal gland has a tumor or is inflamed, CT scanning or MRI imaging could be performed. However, for typical dry-eye syndrome these options would not be reasonable.”
Dr. Hwang acknowledges that this approach has some limitations as a means to diagnose dry eye—at least for now. “First, all of the palpebral lobes of the patient can’t easily be exposed,” he says. “Second, we were only able to obtain cross-sections of a part of the palpebral lobe. Third, current OCT scans of the lacrimal glands can only reveal what is 200 to 400 µm below the surface of the lobule. This is a function of the OCT technology. In our experiments we used the Spectralis OCT, which has a central laser wavelength of 840 µm. On the other hand, the central wavelength of the laser of the Visante OCT is 1,300 µm. If the Visante OCT were used for lacrimal gland scanning, the resolution would be lower but the image depth would increase.”
So far, although differences between healthy and diseased eyes are apparent, the scans have not allowed researchers to conclusively identify which subjects have dry eye and which do not. The team believes this is the result of the shallow depth and low resolution they’ve been able to obtain so far, using current versions of OCT. Dr. Hwang says the researchers will continue gathering images and working to determine which parameters and characteristics in the lacrimal gland distinguish dry-eye syndrome from normal eyes. REVIEW
Dr. Rosenfeld’s group receives research grants from Carl Zeiss Meditec, and the University of Miami has a licensing agreement with Zeiss for some of the algorithms in the Cirrus spectral-domain instrument. Drs. Lam, Wen and Jiao have no current financial interest in VIS-OCT; however Florida International University has filed for a provisional patent, and they are named as the inventors of the technology. Dr. Hwang has no financial connection to any OCT company.
1. Liu T, Wen R, Lam BL, Puliafito CA, Jiao S. Depth-resolved rhodopsin molecular contrast imaging for functional assessment of photoreceptors. Sci Rep 2015;5:13992.
2. Doh SH, Kim EC, Chung SY, et al. Optical Coherence Tomography Imaging of Human Lacrimal Glands: An In Vivo Study. Ophthalmology 2015;122:11:2364-66.



