Not surprisingly, over the years surgeons have developed numerous techniques and devices that help to offset the problems created by a small pupil—usually by temporarily enlarging it. Recently, several new ways to manage small pupils during surgery have become available. Here, surgeons familiar with their use discuss the advantages of each option.
The Malyugin Ring 2.0
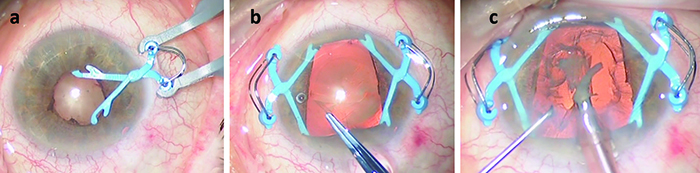
MicroSurgical Technologies (Redmond, Wash.) will be releasing Malyugin Ring 2.0—a new version of the popular Malyugin Ring—in early 2016. Like the existing version, the new model will be available in two sizes, 6.25 and 7 mm, and will be placed inside the eye through the main surgical incision with no need for extra paracenteses. Both the original and new rings, which resemble a square with a loop at each corner, provide eight points of iris fixation, making the pupil very close to round when the device is in place. (See images, top of p. 25.) Each ring comes with a disposable injector, and a manipulator specifically designed for the device is available. The new ring is softer and more elastic than the previous model and comes with a redesigned inserter that can easily fit through a 2-mm clear corneal incision. (The earlier version required a larger incision.)
The designer of the rings, Boris Malyugin, MD, a professor of ophthalmology in the department of cataract and implant surgery at the S. Fyodorov Eye Microsurgery Complex in Moscow, notes that a trend toward the use of smaller incisions has recently been evident in cataract surgery. “The existing version of the Malyugin Ring easily goes through an incision that’s 2.2 mm or bigger,” he notes. “But today, cataract surgery can be done through 1.8- to 2-mm incisions, so the device had to be redesigned to stay in line with the most up-to-date cataract technology.
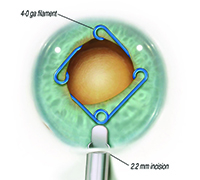 | 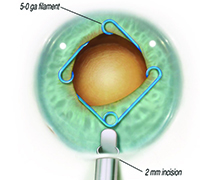 | 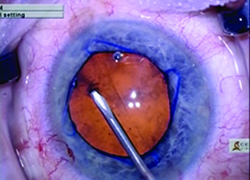 |
| The new Malyugin Ring 2.0 is made of 5-0 prolene instead of 4-0, with a new injector that fits through a smaller incision. (See comparison, left.) Right: the new ring in use. | ||
Robert H. Osher, MD, a professor in the Department of Ophthalmology at the University of Cincinnati College of Medicine, and Medical Director Emeritus at the Cincinnati Eye Institute in Ohio, was the first surgeon to use the new Malyugin Ring 2.0, in December 2014; to date, he’s used about 50 of the new rings. “The original injector had a large finger that required a pretty good vertical excursion,” he notes. “Many surgeons had difficulty with it. The new injector is dramatically different, with a tiny finger that’s very easy to engage and disengage. The injector also was a tight fit in the incision, so the company has made the new version thinner; now, it’s easy to get the injector through a 2-mm incision.” (The new injector will now be included with all of the Malyugin rings.)
“The ring itself is no longer 4-0 prolene; it’s 5-0, and the scrolls are larger,” he continues. “It has greater flexibility. The existing manipulator, which I designed for the earlier generation Malyugin ring, works fine with the new ring. It’s used with your left hand through the side port to help you position, engage and disengage the scrolls.”
 | 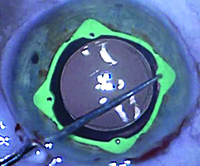 |
| The Visitec I-Ring Pupil Expander engages the iris 360°, creating a uniform circular opening that can be used as a guide during capsulorhexis creation. | |
“Removing the ring is equally easy,” he continues. “I always disengage the distal ring first and let it go into the angle. Then I free the right and left scrolls, and finally disengage the proximal ring. I move it as far toward the center of the pupil as I can. Putting a little Healon 5 around it freezes it in position so it’s easy to put the injector in, capturing the proximal scroll. As I retract the little finger on the injector the ring folds into the injector housing. I use the second manipulator to depress the right and left scrolls onto the platform and they slide into the housing of the injector as well. The distal scroll follows, and I remove the device. It’s easy, safe and simple. I’m certainly happy with it.”
The Visitec I-Ring
The Visitec I-Ring Pupil Expander is an atraumatic, single-use iris expander made of soft, resilient polyurethane. Unlike many approaches to iris expansion, the I-Ring engages the iris completely, expanding it evenly over 360 degrees, creating a uniform, circular opening 6.3 mm in diameter. (The circular shape can then help to guide the creation of a capsulorhexis.)
Although the space created by the ring is circular, the outside of the ring has four “corners” pointing away from the central opening, creating four pockets, or channels, that hold the iris in place. The channels are designed to never distort or pinch the iris, while providing vertical stability for the iris diaphragm and allowing the iris to engage and disengage easily. Each corner contains a positioning hole for a Sinskey hook, isolated from the channel in which the iris sits, to ensure that the Sinskey hook doesn’t touch the iris during placement. The device’s low profile is designed to facilitate the use of microsurgical instruments.
The I-Ring comes packaged in a “nest” with the inserter attached. When the surgeon is ready to use the ring, he retracts the slider on the inserter, causing the ring to fold as it’s pulled into the inserter; the “nest” is then discarded. After injection of viscoelastic, the inserter is positioned inside the eye. Moving the slider forward slowly releases the ring above the iris, where it returns to its functional shape. Placement is accomplished using a Sinskey hook. Insertion, engagement and removal of the device can be done using one hand.
Kenneth R. Kenyon, MD, clinical professor at Tufts University School of Medicine, acts as consulting medical director for Beaver-Visitec International, manufacturer of the device. He was involved with the development of the I-Ring from its inception; since the product has been commercially available, he has used it in both routine small-pupil cataract procedures and more complex surgical scenarios. “I believe the I-Ring represents the next generation of pupil expansion devices,” he says. “It incorporates advances in safety, reliability and consistency.”
 |
| The Assia Pupil Expander uses two spring-loaded devices inserted through 1.1-mm sideport incisions to widen the pupil. The devices can be placed assymetrically, if desired, to create a wider opening close to the surgical incision. |
Roberto Pineda II, MD, director of refractive surgery in the Cornea Service at Massachusetts Eye and Ear in Boston, was also involved in the early design of the I-Ring and has used the product in cataract surgery since its commercial availability in April 2015. “The I-Ring loads effortlessly,” he agrees. “Unlike other pupil expansion rings, it provides three-dimensional stability to the iris. This is key to its ability to deal with cases of intraoperative floppy iris syndrome or trampolining of the iris. It’s an incredibly valuable device for many of our cataract surgery patients.”
You can watch online videos illustrating the use of the Visitec I-Ring at https://www.youtube.com/watch?t=191&v=zzfGIx9AJk0 or at http://www.beaver-visitec.com/products/i-ring-early-clinical-experience.cfm.
The Assia Pupil Expander
The Assia Pupil Expander (aka APX), from APX Ophthalmology in Haifa, Israel, achieves pupil expansion using two tiny spring-loaded devices that are inserted through 1.1-mm sideport incisions opposite each other. (The incisions are generally placed perpendicular to the main incision for the phaco instrument.) A specially designed forceps closes each device for insertion and removal. In the closed position, the curved tips are inserted through the pupil and behind the iris. The device is positioned by the surgeon as the forceps is gently released; when both devices have been inserted and positioned, they create a rectangular pupil opening about 6 x 6 mm. A hook near each tip prevents the shaft from sliding. No intraocular manipulation is required. If desired, the APX devices can be placed asymmetrically, rather than opposite each other, to create a trapezoidal-shaped opening. This allows the surgeon to create a wider opening closer to the surgical incision. (See example, above.) The company notes that the device can’t fall into the vitreous cavity if the surgeon experiences a posterior capsule rupture, unlike rings placed completely inside the eye.
The APX device was designed by Ehud I. Assia, MD, professor of ophthalmology at the Sackler School of Medicine at Tel Aviv University in Israel, and medical director of the Ein Tal Eye Center. “Most current pupil dilators include either iris hooks or pupillary rings,” he notes. “Hooks require four sideport incisions, and they’re time-consuming. The shafts of the hooks outside the eye often extend beyond the surgical field, and occasionally the tips disengage from the pupillary margin and require repositioning during surgery. Rings that can be inserted through the existing surgical opening are quite effective, but they may require considerable intraocular manipulation during insertion and removal. Also, they occupy the entire pupillary margin, which means the surgical instruments must work over some part of the ring.
“I wanted to have a device that would be quick and easy to insert and remove, one that would provide adequate pupil dilation but would not interfere with surgery,” he continues. “After trying various models and designs I concluded that a scissor-like configuration with blunt, rounded tips and an external spring would be the most efficient and practical. The first generation, the reusable metal APX-100, received an FDA 510K exemption in 2013. With feedback from surgeons we’ve now developed the second-generation APX-200, which is disposable. The complete product and package is expected to be ready for use in October 2015.”
Dr. Assia says the APX-200 comes with two designated disposable forceps, allowing the nurse to load and lock the two devices while the surgeon prepares the sideport incisions, thus saving time. “Insertion and removal of the APX only takes a few seconds, and manipulations are entirely extraocular,” he says. “The APX system can be used for superior, lateral or oblique surgical approaches, and it’s been used during vitreoretinal surgery without interfering with the pars-plana vitrectomy instruments.”
Alan Crandall, MD, clinical professor, senior vice chair of ophthalmology and visual sciences and director of glaucoma and cataract at the Moran Eye Center, University of Utah, says he has used the APX pupil expander in cases of intraoperative floppy iris syndrome, uveitis and—most importantly for him—pseudoexfoliation. “Leaving the sub-incisional area open is very helpful when I’m using the Ultra Chopper in very hard cataracts,” he notes. “It also helps in eyes where I would like one side of the pupil to be slightly larger, as in cases of trauma, for example. I also like its broad iris touch that leaves no areas of depigmentation and no iris tears.”
 |
| Omidria is a combination of phenylephrine 1% and keterolac 3% that can be added to the irrigation solution during cataract surgery. It prevents intraoperative pupil constriction and reduces postoperative pain. |
Omidria
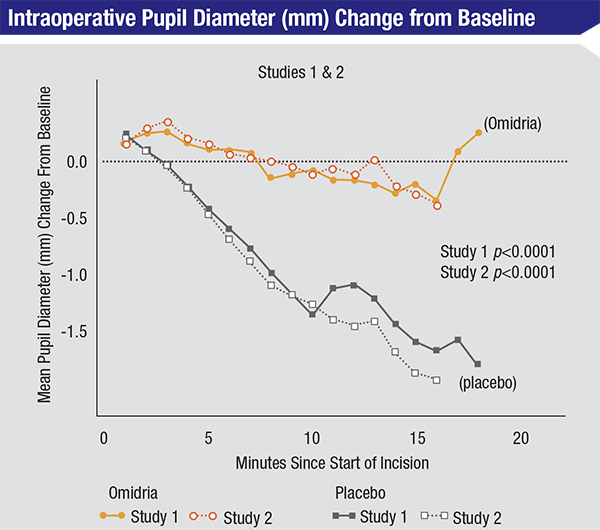
In addition to mechanical devices, a new drug-based tool for managing small pupils during surgery also recently became available. In June 2014 the Food and Drug Administration approved Omeros Corp’s Omidria, a combination of phenylephrine 1% (a pupil-dilating agent) and ketorolac 3% (a non-steroidal anti-inflammatory agent that reduces pain due to surgical trauma and inhibits surgically induced miosis). Omidria is added to the irrigation solution used during cataract surgery and intraocular lens replacement; it’s designed to prevent intraoperative pupil constriction and reduce postoperative pain without requiring any change in the doctor’s surgical routine. In a Phase II trial comparing Omidria to phenylephrine alone, 22 percent of subjects on phenylephrine had a pupil diameter smaller than 6 mm at some point during the surgery; only 6 percent of subjects receiving Omidria did (p=0.0221). (Other trials comparing Omidria to standard cataract surgery protocol found that Omidria also produced statistically significant and clinically meaningful reduction of postoperative pain.)
Dr. Osher, who published a paper on the pharmacology of Omidria coauthored by Ike Ahmed, MD,1 says he’s very impressed with it. “I use it on all of my Flomax patients,” he notes. “I also use it on any pupil which I would say is intermediate size, around 4.5 to 5.5 mm. However, in my experience, Omidria does not add to the pupillary dilatation; it simply prevents the pupil from constricting—a very important function. In busy practices where they try to dilate the patient very quickly, it may add to the dilatation. But in my practice, patients are in the preop area for a long time; they get lots of drops and gels and their pupils are maximally dilated. So, I find Omidria most beneficial when I’m working with a mid-sized pupil or a Flomax case, in concert with viscomydriasis using Healon 5.
“If the pupil is 4 mm or smaller, I prefer to use a Malyugin ring,” he continues. “The ring takes care of widening the pupil. In theory, the ketorolac in Omidria might reduce the inflammation that one may see when manipulating the iris, but I think the Malyugin Ring is very gentle. In fact, my eyes look incredibly quiet when I use the Malyugin ring.”
Richard L Lindstrom, MD, managing partner in Minnesota Eye Consultants and an attending surgeon at the Phillips Eye Institute and Minnesota Eye Laser and Surgery Center in Minneapolis, agrees that Omidria is designed to maintain dilation, not cause it. “Omidria is not labeled for ‘creating’ dilation of the pupil,” he notes. “So, if a pupil is not large enough to safely remove a cataract, in the surgeon’s opinion, pupil expanders are needed to create a larger pupil. If the pupil size at the beginning of the case seems adequate, Omidria will help maintain the pupillary dilation during surgery and reduce intraoperative miosis. It will also improve intraoperative and early postoperative patient comfort.” (Dr. Lindstrom adds that he finds epi-lidocaine or epi-Shugarcaine injected into the anterior chamber at the beginning of surgery to be another useful adjunct.)
Small Pupil? No Problem.
“There have been a lot of small-pupil techniques over the years,” notes Dr. Osher. “I’ve seen them all. They all have been very helpful, because regardless of what you do, when you enlarge the pupil, you make the surgery safer, easier and more enjoyable for the surgeon, and you get better patient outcomes. So it’s worth spending the extra few moments it takes to manage the pupil.” REVIEW
Dr. Pineda is a consultant for Beaver-Visitec International. Dr. Lindstrom is a consultant for Omeros. Dr. Osher is a consultant for MicroSurgical Technologies and Omeros but has no financial interest in any product discussed in the article.
1. Osher RH, Ahmed IK, Demopulos GA. OMS302 (phenylephrine and ketorolac injection) 1%/0.3% to maintain intraoperative pupil size and to prevent postoperative ocular pain in cataract surgery with intraocular lens replacement. Expert Review of Ophthalmology 2015:10;2;91-103.



