|
Size and Design Advances
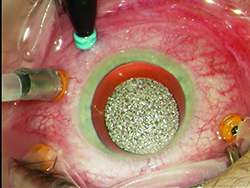
Modern systems utilize various gauges including 23 ga., 25 ga. and 27 ga., and are available as a trocar/cannula system. Advances have been made with better instrumentation and wound-construction techniques to minimize the risk of wound leakage, hypotony, and endophthalmitis. Sclerotomy wound construction techniques involve trocar entry made at an oblique angle to lengthen the wound and maximize the chance of spontaneous closure. The trocars themselves have been redesigned to result in easier insertion and smaller wound profiles that are more likely to self-seal. If there is evidence of leakage at the conclusion of surgery one should suture the leaking sclerotomy to minimize postop complications.
A relatively new addition to the trocar system is the addition of valves, which allow vitrectomy surgery in a closed system, minimizing leakage of fluid during instrument exchange, reducing the potential for vitreous/retina incarceration and minimizing intraoperative intraocular pressure fluctuations. The latter may decrease the risk of hemorrhage during diabetic dissection; prevent hypotony during vitrectomy surgery, avoiding suprachoroidal hemorrhage and reducing the flow of fluid during surgery; and minimize turbulence (i.e., having fish eggs during perfluoron injection). It is important to note that when extra fluid such as perfluoron or silicone oil is injected into the eye while valved trocars are used, a drainage mechanism (i.e., vent or suction with cutter/soft-tip cannula) must be provided to avoid increased intraocular pressure and optic nerve/retina hypo-perfusion during surgery.
Cutter design itself has been revised to improve its surgical utility. Larger port areas have been employed to hasten vitreous removal. Further, the port itself has been migrated closer to the tip of the cutter, enhancing stability when cutting near mobile retina and in many cases allowing it to be used as a dissecting instrument, as well.
Cutter speed has also been increased through different mechanisms. Higher cut rates reduce the size of each individual “bite” of vitreous, which in turn decreases the cutter-induced vitreoretinal traction during vitrectomy. This may be important during maneuvers such as removal of vitreous near a detached retina, minimizing the risk of iatrogenic retinal breaks.
Duty cycle is another variable that can be adjusted by the surgeon during the vitrectomy surgery, independent of the cut rate. It refers to the percentage of time the cutter port remains open during each cutting cycle, and the surgeon may choose between open or closed bias. When operating at close proximity to the retina, the closed bias decreases the likelihood of tissue incarceration, that is during peripheral vitreous shaving or fibrous tissue dissection. On the other hand, the open biased mode would allow a greater flow of fluid, such as during core vitrectomy without needing to change the cut rate.
In the future, smaller instruments such as 27-ga. trocar systems that provide superior fluidics and minimize surgical trauma will likely become more widely adopted. Such systems further reduce the the need for suturing; reduce surgical trauma; improve postop recovery; and lead to faster visual recovery.
| ||||
Better Visualization
Visualization during vitrectomy surgery has significantly improved over recent years. The two main components that contribute to this improvement are better viewing systems and improved lighting.
Newer non-contact wide-angle viewing systems have optics similar to indirect ophthalmoscopy. The panoramic view offers more than 100 degrees of field of view, allowing the visualization of peripheral retina and vitreous with minimal need to rotate or tilt the eye during vitrectomy surgery.
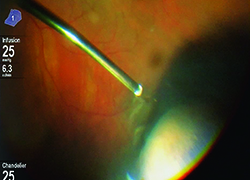
Higher lumen light sources have been developed to compensate for the lower amount of light transmitted through smaller-gauge instruments. These sources are more powerful and can be combined with special filters to minimize the risk of retinal phototoxicity from shorter wavelength light. Furthermore, chandelier light systems have freed up the hands of surgeons, allowing them to perform peripheral vitreous shaving without the need of an assistant, and bimanual dissection techniques in complex diabetic or proliferative vitreoretinopathy patients. It is important to be aware of the potential for phototoxicity and to reduce retinal light exposure to the minimum needed.
Illumination will continue to improve with the use of brighter illuminators, with possible directional and smaller-gauge chandelier lights. Further, chandelier lights could be repositioned easily to illuminate different locations of the globe. On the other hand, better small-gauge illuminating instruments may one day obviate the need for chandelier lights altogether.
The most revolutionary change in the future will probably be the use of optical coherence tomography during vitrectomy surgery. In much the same way that OCT revolutionized our clinical practice in the office, it will probably also revolutionize retinal surgery. The current state of technology for intraoperative use is at an early stage, similar to the time when OCT first became available for office use. Down the road, intraoperative OCT will probably look very different.
Another potentially revolutionary change would be the application of smart technology in the operating room. It would have an impact on every aspect of the surgery, from the time the surgery is scheduled, to the moment patient enters the operating suit (or the hospital) until the moment the patient leaves. Electronic health records are only a small part of this communication pathway. Much of the equipment and devices used for patient care during surgery would be able to communicate to make surgery safer and more efficient. Finally, instrument quality will continue to improve. This includes increasing the rigidity of small-gauge instruments to prevent bending or breakage during vitrectomy, especially when operating in the peripheral retina. REVIEW
Dr. McClintock is the senior fellow in vitreoretinal surgery at the Rush University Medical Center and Illinois Retina Associates in Chicago. Dr. Rezaei is an associate professor of ophthalmology at Rush University Medical Center and senior partner at Illinois Retina Associates. Contact Dr. Rezaei at Illinois Retina Associates, 71 W. 156th St., Ste. 400, Harvey, IL 60426. Phone: (708) 596-8710; fax: (708) 596-9820; email: karezaei@yahoo.com.
1. Savastano A, et al. Combining cataract surgery with 25-gauge high-speed pars plana vitrectomy: Results from a retrospective study. Ophthalmology 2014;121:299-304.
2. Kumar A., et al. Comparative evaluation of 23- and 25-gauge microincision vitrectomy surgery in management of diabetic macular traction retinal detachment. Eur J Ophthalmol 2014;24(1):107-13.
3. Diniz B, et al. Fluidics in a dual pneumatic ultra high-speed vitreous cutter system. Ophthalmologica 2013;229(1):15-20.
4. Diniz B, et al. Analysis of a 23-gauge ultra high-speed cutter with duty cycle control. Retina 2013;33: 933-8.
5. Yamane S, et al. Evaluation of microincision vitrectomy wounds made with microvitreoretinal blade or beveled trocar by swept source optical coherence tomography. Retina 2012;32:140-5.
6. Sabti K, Raizada S. Endoscope-assisted pars plana vitrectomy in severe ocular trauma. Br J Ophthalmol 2012;96(11):1399-403.
7. Thompson J. Advantages and limitations of small gauge vitrectomy. Surv Ophthalmol 2011;56(2):162-72.
8. Sakaguchi H, et al. A 29/30-gauge dual-chandelier illumination system for panoramic viewing during microincision vitrectomy surgery. Retina 2011;31:1231-3.
9. Ho L, et al. Study of intraocular pressure after 23-gauge and 25-gauge pars plana vitrectomy randomized to fluid versus air fill. Retina 2011;31:1109-17.
10. Fabian I, Moisseiev J. Sutureless vitrectomy: Evolution and current practices. Br J Ophthalmol 2011;95(3):318-24.
11. Teixeira A, et al. Vitreoretinal traction created by conventional cutters during vitrectomy. Ophthalmology 2010;117:1387-92 e2.
12. Oshima Y, et al. A 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology 2010;117:93-102 e2.
13. Inoue M, Shinoda K, Hirakata A. Twenty-three gauge cannula system with microvitreoretinal blade trocar. Br J Ophthalmol 2010;94(4):498-502.
14. Ho L, Walsh M, Hassan T. 25-Gauge pars plana vitrectomy for retained lens fragments. Retina 2010;30:843-9.
15. Hubschman J, et al. Effect of cutting phases on flow rate in 20-, 23-, and 25-gauge vitreous cutters. Retina 2009;29:1289-93.
16. Shah C, et al. Short-term outcomes of 25-gauge vitrectomy with silicone oil for repair of complicated retinal detachment. Retina 2008;28:723-8.
17. Oshima Y, et al. Novel mercury vapor illuminator combined with a 27/29-gauge chandelier light fiber for vitreous surgery. Retina 2008;28:171-3.
18. Magalhaes Jr O, et al. Vitreous dynamics: vitreous flow analysis in 20-, 23-, and 25-gauge cutters. Retina 2008;28:236-41.
19. Lai M, et al. Repair of primary rhegmatogenous retinal detachment using 25-gauge transconjunctival sutureless vitrectomy. Retina 2008;28:729-34.
20. Gupta O, et al. Short-term outcomes of 23-gauge pars plana vitrectomy. Am J Ophthalmol 2008;146:193-197.
21. Eckardt C, Eckert T, Eckardt U. 27-gauge Twinlight chandelier illumination system for bimanual transconjunctival vitrectomy. Retina, 2008;28:518-9.
22. DeBoer C, et al. Port geometry and its influence on vitrectomy. Retina 2008;28:1061-7.
23. Oshima, Y., C.C. Awh, and Y. Tano, Self-retaining 27-gauge transconjunctival chandelier endoillumination for panoramic viewing during vitreous surgery. Am J Ophthalmol 2007;143: 166-167.
24. Lewis H. Sutureless microincision vitrectomy surgery: Unclear benefit, uncertain safety. Am J Ophthalmol 2007;144:613-5.
25. Gupta O, et al. Postoperative complications associated with 25-gauge pars plana vitrectomy. Ophthalmic Surg Lasers Imaging 2007;38(4):270-5.
26. Ibarra M, et al. Longer-term outcomes of transconjunctival sutureless 25-gauge vitrectomy. Am J Ophthalmol 2005;139:831-6.
27. Eckardt C. Transconjunctival sutureless 23-gauge vitrectomy. Retina 2005;25:208-11.
28. Charles S. An engineering approach to vitreoretinal surgery. Retina 2004;24:435-44.
29. Peyman G, et al. A new wide-angle endoillumination probe for use during vitrectomy. Retina 2002;22:242.
30. Fujii G, et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology 2002;109:1807-12; discussion 1813.
31. Fujii G, et al. Initial experience using the transconjunctival sutureless vitrectomy system for vitreoretinal surgery. Ophthalmology 2002;109:1814-20.
32. van den Biesen P., et al. Endoillumination during vitrectomy and phototoxicity thresholds. Br J Ophthalmol 2000;84(12):1372-5.