Despite the vast amount of surgical experience accumulated since the advent of vitrectomy surgery in the 1970s, complications are still a part of vitreoretinal surgery. Postoperative elevated intraocular pressure, cataract formation and endophthalmitis are complications that can occur after all intraocular surgery, including vitreoretinal surgery. A number of complications, however, are unique to vitreoretinal surgery. This review is not intended to thoroughly describe every possible vitreoretinal surgical complication, but will briefly highlight several of the complications specific to vitreoretinal surgery, including their prevention and management.
Retrobulbar Anesthesia Complications
Though most cataract surgeons today perform cataract extraction under topical anesthesia, vitreoretinal surgeons still rely heavily on either general anesthesia or the retrobulbar block. Though rare, retrobulbar anesthesia complications can occur (See Figure 1). One review reported a 0.08 percent incidence of globe penetration in 4,000 patients who underwent retrobulbar anesthesia for retinal reattachment surgery.1 In all of the cases, high axial myopia was a significant contributing factor. Another group estimated that the incidence of perforation from a retrobulbar block may be as high as 0.71 percent in eyes with an axial length of greater than or equal to 26 mm.2 The most common complication following inadvertent globe perforation is vitreous hemorrhage, which has been found to occur in up to 85 percent of all cases.3 An associated retinal detachment occurs nearly 50 percent of the time, and is usually associated with poor final visual acuity. One study found that 78 percent of patients with an associated retinal detachment from inadvertent globe penetration secondary to retrobulbar anesthesia had a final visual acuity of counting fingers or worse, most of which had resulted in complex retinal detachments and proliferative vitreoretinopathy requiring multiple surgeries.3 Because of the high associated incidence of retinal detachment in these cases, early intervention with vitrectomy is recommended when the view to the fundus is obscured by vitreous hemorrhage secondary to accidental globe perforation.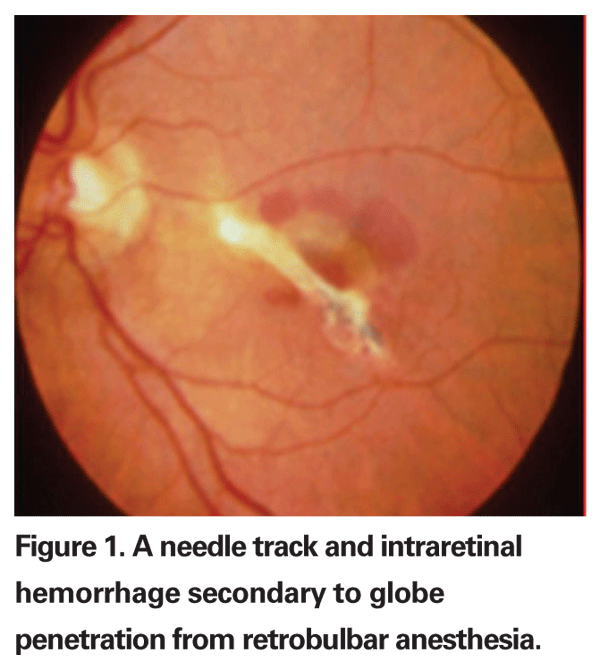
In addition to globe penetration, complications of retrobulbar anesthesia include accidental injection of anesthesia into or around the optic nerve sheath. As a result, retrobulbar hemorrhage, optic neuropathy and retinal vascular occlusions can occur.4 Retinal vascular occlusions include both central artery occlusions as well as combined central artery and vein occlusions, leading to significant permanent visual loss.5-8 A report of three patients who had combined central retinal artery and vein occlusions following retrobulbar anesthesia found a dilated optic nerve sheath in all cases via B-scan ultrasonography or computed tomography.7 Another group confirmed this finding by magnetic resonance imaging as well.9 This suggests that the likely underlying pathogenetic mechanism is optic nerve sheath compression either from the anesthetic itself or from localized hemorrhage.
Beyond the ocular complications associated with inadvertent injection of anesthesia into the optic nerve sheath, even more dangerous central nervous system disturbances can occur as well. Dysarthria, grand mal seizures and even respiratory arrest have been documented following retrobulbar anesthesia.10-14 It is thought that the anesthesia reaches the brainstem quickly through the central retinal artery or in a more delayed fashion via the optic nerve sheath itself.
We recommend a more controlled procedure when performing retrobulbar anesthesia, by first sedating the patient with fentanyl and midazolam (Versed), followed by a bolus of intravenous propofol. This is followed by creating a small puncture in the conjunctiva and Tenon's of the inferior fornix and injecting a 50:50 mixture of lidocaine and bupivicaine in the sub-Tenon's space using a 1-inch curved blunt irrigating cannula. This technique has been found to have both excellent safety and efficacy.15,16 Not only does this prevent globe penetration, but the delivery of the anesthesia into the correct anatomic space can be visually observed from start to finish.
Suprachoroidal Hemorrhage
One of the more feared complications for all eye surgeons is an intraoperative massive suprachoroidal hemorrhage. The incidence of suprachoroidal hemorrhage during vitrectomy surgery has been estimated to be 0.17 to 1.9 percent.17-20 Risk factors for a suprachoroidal hemorrhage during vitreoretinal surgery include advanced age, elevated preoperative IOP, aphakia, pseudophakia, scleral buckling, high myopia, external drainage of subretinal fluid, cryotherapy, coughing or "bucking" on the endotracheal tube during general anesthesia, and intraoperative systemic hypertension.18,19,21,22 The underlying pathophysiologic mechanism causing massive suprachoroidal hemorrhage is likely secondary to acute intraoperative ocular hypotony. If the IOP is significantly lowered in a short period of time, especially if it is initially elevated, the short or long posterior arteries may rupture, giving rise to a severe suprachoroidal hemorrhage. This can lead to even more bleeding as the forced separation of the choroid away from the sclera will avulse more blood vessels.23 Large IOP fluctuations can often occur during vitreoretinal surgery, such as during aspiration of subretinal fluid, phacofragmentation and inadvertent needle penetration of the globe during scleral buckle placement.24 Additionally, the choroid itself can be directly traumatized by instruments during internal drainage of subretinal fluid and removal of subretinal proliferative membranes, which can result in localized choroidal hematomas.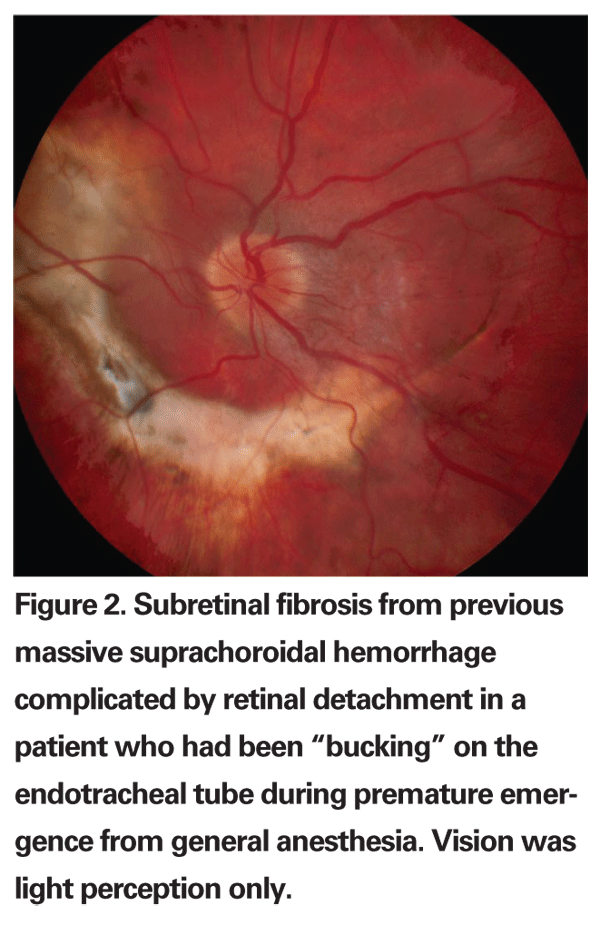
Intraoperative management of suprachoroidal hemorrhage is aimed primarily at stabilizing the IOP and preventing extrusion of intraocular contents. Increasing the IOP to 60 mmHg or more can usually be instantaneously accomplished by depressing a pre-determined switch on the vitrectomy foot pedal. The infusion line should also be checked frequently to make sure that the infusion cannula has not entered the subretinal space. Drainage posterior sclerotomies can be considered in more severe cases or when intraocular tissues are incarcerated. It is often impossible, however, to drain all of the hemorrhage, as there is usually a persistent localized blood clot. Moreover, it does not appear to have any significant beneficial effect on the long-term visual outcome.22,23 Visual outcomes in patients who have had an intraoperative suprachoroidal hemorrhage during vitrectomy surgery are generally poor (See Figure 2). Additionally, there is an increased incidence of retinal detachment, secondary glaucoma and eventual hypotony.23
Iatrogenic Phototoxicity
Iatrogenic macular phototoxicity was first described on six patients who developed a characteristic macular lesion following cataract extraction.25 In these cases, well-circumscribed white lesions involving the outer retina several disc diameters in size were seen in the macula, and within a few weeks, the whitening was gradually replaced by pigmentary mottling of the retinal pigment epithelium. Tungsten filament sources have been found to cause more oval-shaped lesions, whereas fiber optic microscope illuminators usually produce a more homogenous, round lesion.
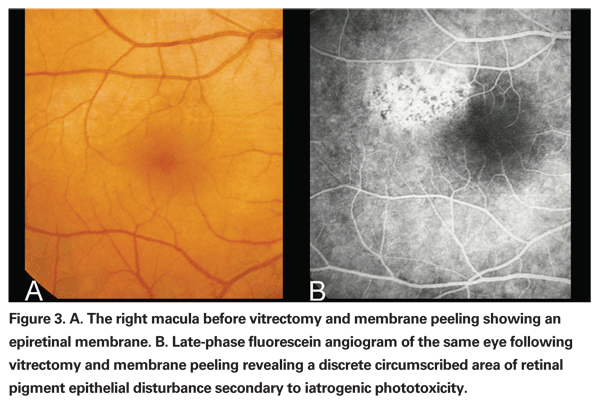
Iatrogenic phototoxicity during vitrectomy has been reported both from the operating microscope as well as the endoilluminator (See Figure 3).26,27 Phototoxic lesions caused by the endoilluminator are similar to lesions caused by the operating microscope but are usually larger in size and have less well-delineated borders.27 The wavelength of light used, the power of the light source and the duration of use determine the amount of ocular damage in phototoxicity. One study with long-term follow up of patients with iatrogenic phototoxicity following either cataract or vitrectomy surgery found the average duration of surgery to be 109 minutes.28 Numerous animal studies have also demonstrated the increased efficiency of phototoxicity when using wavelengths less than 515 nm.29-31 Exposure to the operating microscope light should be minimized as much as possible by either turning off the illumination source or placing a small shield or cover on the cornea when not using the microscope. The intensity of the light source should also be titrated only to the level that is sufficient for the surgeon to accomplish the procedure and no higher. This is especially important in regards to the recent advent of xenon and mercury vapor light sources, where inadvertent exposure to potentially extremely high light intensities is likely to increase the incidence of iatrogenic phototoxicity.
Nitrous Oxide
In patients with pre-existing intraocular gas bubbles that have undergone vitreoretinal surgery, the use of nitrous oxide during general anesthesia by the anesthesiologist in subsequent surgical procedures can lead to intractable elevation of IOP and an eventual central artery occlusion.32,33 Nitrous oxide is a commonly used general anesthetic agent, and, because of its high solubility, will diffuse quickly into intraocular gas bubbles, whereas gases commonly used for long-term tamponade of the retina, such as perfluoropropane (C3F8) or sulfur hexafluoride (SF6), diffuse slowly into the circulation. This will cause a net rapid expansion of the existing intraocular gas bubble and subsequent elevation of IOP, which in many cases can lead to a central artery occlusion and permanent visual loss (See Figure 4).34 Ventilation with nitrous oxide in cats caused a threefold increase in the volume of an intraocular SF6 bubble and a twofold increase in the volume of an intraocular air bubble.35 In contrast, ventilation with oxygen alone caused only a 35-percent increase in the volume of an intraocular SF6 bubble and a 15-percent decrease in an air bubble. These findings were independently confirmed using a mathematical model, and the authors of the study concluded that if ventilation with nitrous oxide is discontinued at least 15 minutes before the gas-fluid exchange, the volume of gas would only expand by 15 percent. In a series of five patients who had a pre-existing gas bubble from prior retinal surgery and underwent a different surgical procedure involving general anesthesia with nitrous oxide ventilation, four of the five eyes had a final vision of 20/200 or worse and significant optic atrophy. Three eyes had no light perception. It is very important, therefore, to notify the anesthesiologist ahead of time before performing an air-fluid exchange under general anesthesia, to insure that nitrous oxide is not used as an anesthetic. Patients undergoing vitreoretinal surgery requiring intraocular gas bubbles should also wear a special wrist band postoperatively until the gas bubble is completely reabsorbed in order to notify the anesthesiologist if the patient needs a subsequent surgical procedure requiring general anesthesia.
Air-fluid Exchange Complications
A fundamental maneuver in vitreoretinal surgery for repairing retinal detachments, macular holes and any other procedure requiring gas tamponade, is the air-fluid exchange, where fluid in the vitreous cavity is replaced by air. Though the maneuver is typically completed within minutes and usually signals a satisfactory completion of vitrectomy, complications can still occur. One such complication is damage to the retina associated with air infusion.36,37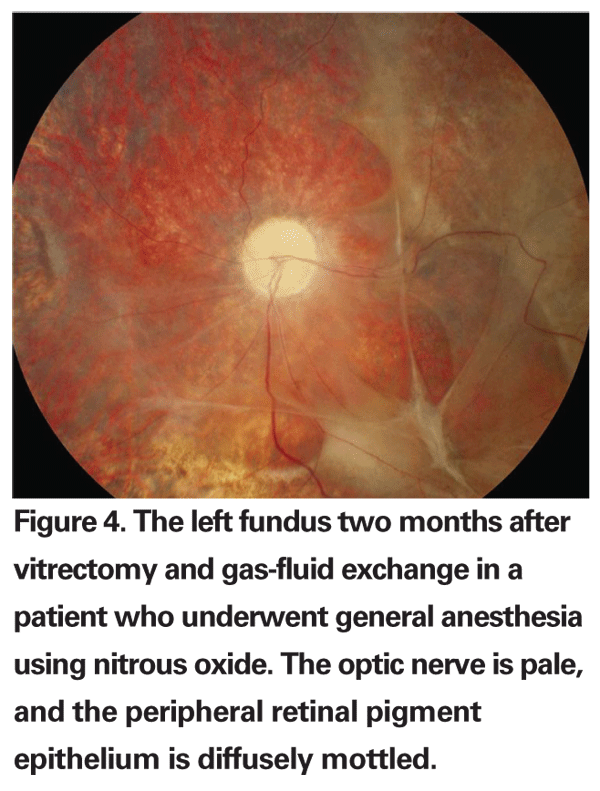
If the IOP immediately preceding the infusion of air is elevated, as can occur iatrogenically with the vitrectomy foot pedal, the pressure of the air infusion can be high enough to cause damage to the retina contralateral to the infusion cannula. With the infusion cannula placed inferotemporally in most cases, damage to the retina from air infusion is usually seen as whitening of the retina in the superonasal quadrant (See Figure 5). One group performed air-fluid exchange in vitrectomized rabbit eyes with an air pressure of 25 and 40 mmHg for 30 seconds.38 They found inner retinal damage as well as focal detachment of the retina and adhesion of RPE cells to photoreceptor cells by scanning electronic and light microscopy. No morphological changes were seen in rabbit eyes that underwent vitrectomy alone.
Another complication during air-fluid exchange is mechanical trauma to the optic nerve head during aspiration. Three patients in one report underwent vitrectomy with air-fluid exchange and subsequently developed permanent peripheral temporal visual field defects.39 The authors felt that in each case the optic nerve head underwent mechanical trauma during aspiration with the extrusion instrument. Another study reported on patients with visual field defects after receiving air-fluid exchange and observed optic disc pallor and afferent pupillary defects, further suggesting trauma to the optic nerve as a mechanism for visual field loss.40 Using a soft-tip extrusion cannula under clear visualization during air-fluid exchange will help insure that mechanical trauma is minimized.
Retained Perfluorocarbon Liquid
Perfluorocarbon liquids are a commonly used surgical adjunct in the management of retinal detachment repair, particularly when dealing with proliferative vitreoretinopathy or giant retinal tears. Because of their high specific gravity and weight, injection of perfluorocarbon liquids into the posterior chamber will flatten detached retina against the RPE and displace fluid anteriorly through any peripheral breaks, as well as provide counter-traction when peeling epiretinal membranes.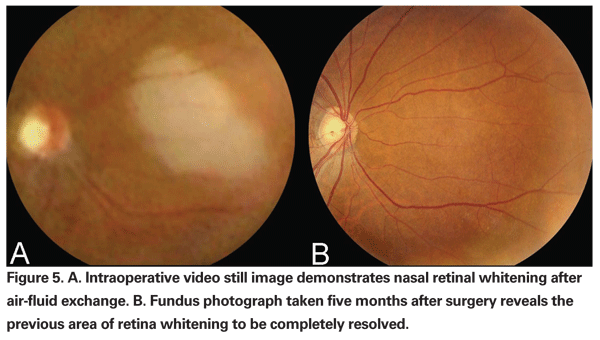
Surgical complications from perfluorocarbons are rare but can occur and are the result of retention postoperatively with subsequent toxicity. Reported toxicities include secondary glaucoma from retained intraocular perfluorocarbon as well as central scotomas from retained subfoveal perfluorocarbon.41,42 Retinal pigment epithelial and photoreceptor toxicity was demonstrated in an animal model with retained subretinal perfluorocarbon liquid.43 Other research demonstrated stromal inflammation, loss of endothelial cells and corneal vascularization in aphakic rabbit eyes that were injected with perfluorocarbon liquids.44 Risk factors for retained subretinal perfluorcarbon include large peripheral retinotomies greater than 120 degrees, as well as lack of a saline rinse after perfluorocarbon liquid removal.45 Performing a saline rinse after removal of perfluorocarbon liquid likely helps collect a microscopic residual layer of perfluorcarbon liquid posteriorly that can then be aspirated.
Sympathetic Ophthalmia
An unusual complication that occurs rarely but has been reported after vitrectomy surgery is sympathetic ophthalmia. A bilateral diffuse granulomatous uveitis occurring in patients who have sustained previous ocular trauma or surgery, sympathetic ophthalmia has also been reported in patients following vitrectomy.46 Most patients have a history of antecedent trauma such as a ruptured globe, but cases have been reported after primary vitrectomies, as well.47 The incidence of sympathetic ophthalmia following vitrectomy has been estimated to be 0.01 percent,46 similar to the incidence of 0.007 percent reported for postsurgical sympathetic ophthalmia.48
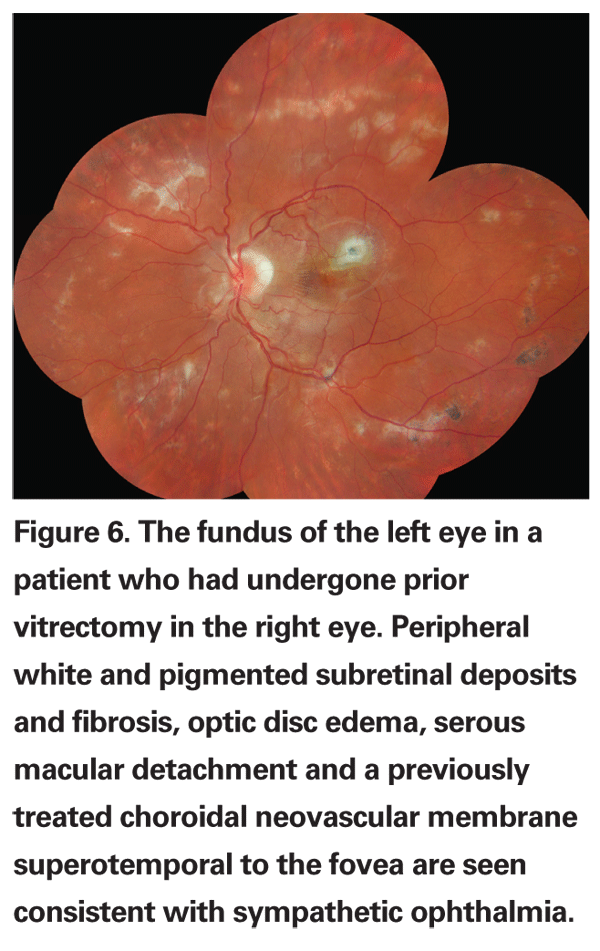
Sympathetic ophthalmia tends to occur between three weeks and six months following vitrectomy and is characterized by bilateral anterior chamber inflammation, vitritis, Dalen-Fuchs nodules and exudative serous retinal detachment (See Figure 6). Long-term complications include patchy or diffuse retinal pigment epithelial atrophy and rarely, choroidal neovascularization.
Therapy for sympathetic ophthalmia includes enucleation and immunosuppression. The role of enucleation in the therapy of sympathetic ophthalmia is controversial. Some advocate early enucleation, specifically within two weeks after surgery,49 while others contend that the timing of enucleation has no effect on the final visual outcome.50 Oral prednisone is the most common and first-line agent, but long-term control usually requires use of one or more immunosuppressive agents, such as methotrexate, azothioprine, cyclosporine or chlorambucil.
Wound Leakage Complications
The introduction of 25-ga. sutureless vitrectomy in 200251 has led to a dramatic increase in the number of small-gauge procedures performed annually by vitreoretinal surgeons. Though heralded as decreasing operating times and increasing patient comfort, the new procedure's complications, such as endophthalmitis, appear to be increased compared to the more "standard" 20-ga. vitrectomy system.52 A retrospective comparison of the incidence of endophthalmitis in more than 8,000 patients undergoing either 25- or 20-ga. procedures found a 12-fold increased risk in the 25-ga. vitrectomy group.52 This suggests that an increased risk of endophthalmitis in patients undergoing small-gauge vitrectomy may be related to inferior wound closure when compared to sutured 20-ga. sclerotomy incisions.
One of the main concerns with small-gauge sutureless vitrectomy is the potential complication of wound leakage and hypotony. A report on 140 consecutive cases using a 25-ga. vitrectomy system found additional suturing of the sclerotomy site was required in 7.1 percent of all cases.53 Another group reported on 70 consecutive patients undergoing 25-ga. vitrectomy and found comparable rates of postoperative hypotony.54 Moreover, fluid-filled eyes had a significantly higher incidence of postop hypotony, as well as other complications such as cataract formation, retinal detachment and cystoid macular edema, compared to eyes with air or gas tamponade.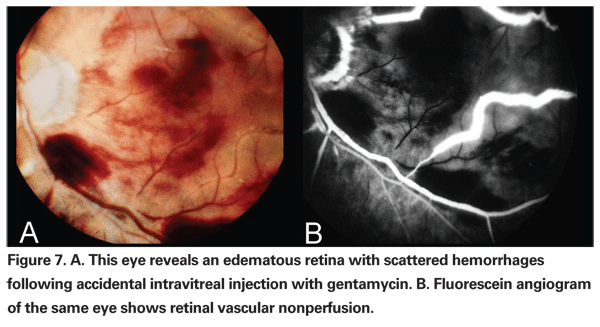
Several authors have demonstrated superior wound closure using a 25-ga. vitrectomy system by creating tunneled oblique incisions in the sclera as opposed to perpendicular insertion of the trocars.55,56 We recommend creating oblique scleral tunneled incisions when inserting the cannulas for 25-ga. vitrectomy, as well as performing a partial air-fluid exchange in cases where gas tamponade is not required. In addition, the threshold for placing an additional suture in a small-gauge incision should be low. Any hint of bleb formation or wound leakage should prompt the placement of a suture by the surgeon.
Aminoglycoside Toxicity
Lastly, a potentially catastrophic but preventable complication is the accidental injection of aminoglycoside antibiotic into the vitreous cavity. Though less commonly used today, aminoglycosides are still used intravitreally for the treatment of endophthalmitis as well as subconjunctivally for antibiotic prophylaxis. Aminoglycosides have been reported to cause an acute, toxic ischemic retinopathy in high concentrations.57 Toxicity from aminoglycosides is characterized by severe retinal vascular occlusion and optic neuropathy, often with permanent severe visual loss (See Figure 7). Injection of aminoglycosides subconjunctivally has been reported to cause toxicity as well.58 It is important that all syringes be precisely labeled, and that all injectable fluids be drawn up only at the time of use; ideally, this should be done under the observation of the surgeon. Additionally, we recommend that aminoglycosides not be used as a routine prophylactic antibiotic following vitrectomy surgery. Suitable antibiotics include cefazolin, or if the patient is allergic to penicillin, vancomycin instead.
Like all ocular surgery, vitreoretinal surgery may have complications, some of which can be devastating. However, the majority of complications are preventable with careful planning and decision making. Having an open dialogue with the anesthesiologist before surgery begins to discuss the specific needs required of vitreoretinal surgery will prevent unwanted patient movement during critical maneuvers as well the use of nitrous oxide when performing an air-fluid exchange. Other preventable measures include minimizing exposure to the operating microscope light or endoilluminator and being aware of the intraocular infusion pressure when performing an air-fluid exchange. Aminoglycosides should probably be avoided as a prophylactic antibiotic at the end of the case. Lastly, the surgical team should always label all intraocular fluids used prior to the start of surgery and these should be double-checked by the surgeon before use.
Drs. Roe and McDonald practice at the San Francisco Retina Foundation and the Pacific Vision Foundation,
1. Ramsay RC, Knobloch WH. Ocular perforation following retrobulbar anesthesia for retinal detachment surgery. Am J Ophthalmol 1978;86:61-4.
2. Duker JS, Belmont JB, Benson WE, et al. Inadvertent globe perforation during retrobulbar and peribulbar anesthesia. Patient characteristics, surgical management, and visual outcome. Ophthalmology 1991;98:519-26.
3. Wearne MJ, Flaxel CJ, Gray P, et al. Vitreoretinal surgery after inadvertent globe penetration during local ocular anesthesia. Ophthalmology 1998;105:371-6.
4. Morgan CM, Schatz H,
5. Klein ML, Jampol LM, Condon PI, et al. Central retinal artery occlusion without retrobulbar hemorrhage after retrobulbar anesthesia. Am J Ophthalmol 1982;93:573-7.
6. Brod RD. Transient central retinal artery occlusion and contralateral amaurosis after retrobulbar anesthetic injection. Ophthalmic Surg 1989;20:643-6.
7. Sullivan KL, Brown GC, Forman AR, et al. Retrobulbar anesthesia and retinal vascular obstruction. Ophthalmology 1983;90:373-7.
8. Mieler WF, Bennett SR, Platt LW, Koenig SB. Localized retinal detachment with combined central retinal artery and vein occlusion after retrobulbar anesthesia. Retina 1990;10:278-83.
9. Horton JC, Hoyt WF, Foreman DS, Cohen JA. Confirmation by magnetic resonance imaging of optic nerve injury after retrobulbar anesthesia. Arch Ophthalmol 1996;114:351-3.
10. Rosen WJ. Brainstem anesthesia presenting as dysarthria. J Cataract Refract Surg 1999;25:1170-1.
11. Meyers EF, Ramirez RC, Boniuk I. Grand mal seizures after retrobulbar block. Arch Ophthalmol 1978; 96:847.
12. Rodman DJ, Notaro S, Peer GL. Respiratory depression following retrobulbar bupivacaine: Three case reports and literature review. Ophthalmic Surg 1987;18:768-71.
13. Castillo A, Lopez-Abad C, Macias JM, Diaz D. Respiratory arrest after 0.75% bupivacaine retrobulbar block. Ophthalmic Surg 1994;25:628-9.
14. Wittpenn JR, Rapoza P, Sternberg P Jr., et al. Respiratory arrest following retrobulbar anesthesia. Ophthalmology 1986;93:867-70.
15. Friedberg MA, Spellman FA,
16. Wald KJ, Weiter JJ. Modified technique of blunt cannula retrobulbar anesthesia for vitreoretinal surgery. Ophthalmic Surg 1993;24:336-8.
17. Speaker MG, Guerriero PN, Met JA, et al. A case-control study of risk factors for intraoperative suprachoroidal expulsive hemorrhage. Ophthalmology 1991;98:202-9; discussion 10.
18. Sharma T, Virdi DS, Parikh S, et al. A case-control study of suprachoroidal hemorrhage during pars plana vitrectomy. Ophthalmic Surg Lasers 1997;28:640-4.
19. Hawkins WR, Schepens CL. Choroidal detachment and retinal surgery. Am J Ophthalmol 1966;62:813-9.
20. Piper JG, Han DP, Abrams GW, Mieler WF. Perioperative choroidal hemorrhage at pars plana vitrectomy. A case-control study. Ophthalmology 1993;100: 699-704.
21. Tabandeh H, Sullivan PM, Smahliuk P, et al. Suprachoroidal hemorrhage during pars plana vitrectomy. Risk factors and outcomes. Ophthalmology 1999;106:236-42.
22. Pollack AL, McDonald HR, Ai E, et al. Massive suprachoroidal hemorrhage during pars plana vitrectomy associated with Valsalva maneuver. Am J Ophthalmol 2001;132:383-7.
23. Tabandeh H, Flynn HW Jr. Suprachoroidal hemorrhage during pars plana vitrectomy. Curr Opin Ophthalmol 2001;12:179-85.
24. Brown P, Chignell AH. Accidental drainage of subretinal fluid. Br J Ophthalmol 1982;66:625-6.
25. McDonald HR, Irvine AR. Light-induced maculopathy from the operating microscope in extracapsular cataract extraction and intraocular lens implantation. Ophthalmology 1983;90:945-51.
26. McDonald HR, Harris MJ. Operating microscope-induced retinal phototoxicity during pars plana vitrectomy. Arch Ophthalmol 1988;106:521-3.
27. Michels M, Lewis H, Abrams GW, et al. Macular phototoxicity caused by fiberoptic endoillumination during pars plana vitrectomy. Am J Ophthalmol 1992; 114:287-96.
28. Postel EA, Pulido JS,
29. Ham WT Jr., Mueller HA, Ruffolo JJ, Jr., Clarke AM. Sensitivity of the retina to radiation damage as a function of wavelength. Photochem Photobiol 1979;29:735-43.
30. Lawwill T. Three major pathologic processes caused by light in the primate retina: A search for mechanisms. Trans Am Ophthalmol Soc 1982;80:517-79.
31. Buyukmihci N. Photic retinopathy in the dog. Exp Eye Res 1981;33:95-109.
32. Sabates WI, Abrams GW,
33. Fu AD, McDonald HR, Eliott D, et al. Complications of general anesthesia using nitrous oxide in eyes with preexisting gas bubbles. Retina 2002;22:569-74.
34. Wolf GL, Capuano C, Hartung J. Nitrous oxide increases intraocular pressure after intravitreal sulfur hexafluoride injection. Anesthesiology 1983;59:547-8.
35. Wolf GL, Capuano C, Hartung J. Effect of nitrous oxide on gas bubble volume in the anterior chamber. Arch Ophthalmol 1985;103:418-9.
36. Yang SS, McDonald HR, Everett AI, et al. Retinal damage caused by air-fluid exchange during pars plana vitrectomy. Retina 2006;26:334-8.
37. Welch JC. Dehydration injury as a possible cause of visual field defect after pars plana vitrectomy for macular hole. Am J Ophthalmol 1997;124:698-9.
38. Hasumura T, Yonemura N, Hirata A, et al. Retinal damage by air infusion during vitrectomy in rabbit eyes. Invest Ophthalmol Vis Sci 2000;41:4300-4.
39. Melberg NS, Thomas MA. Visual field loss after pars plana vitrectomy with air/fluid exchange. Am J Ophthalmol 1995;120:386-8.
40. Kerrison JB, Haller JA, Elman M, Miller NR. Visual field loss following vitreous surgery. Arch Ophthalmol 1996;114:564-9.
41. Lesnoni G, Rossi T, Gelso A. Subfoveal liquid perfluorocarbon. Retina 2004;24:172-6.
42. Foster RE, Smiddy WS, Alfonso EC, Parrish RK, 2nd. Secondary glaucoma associated with retained perfluorophenanthrene. Am J Ophthalmol 1994;118:253-5.
43. de Queiroz JM, Jr., Blanks JC, Ozler SA, et al. Subretinal perfluorocarbon liquids. An experimental study. Retina 1992;12(3 Suppl):S33-9.
44. Moreira H, de Queiroz JM, Jr.,
45. Garcia-Valenzuela E, Ito Y, Abrams GW. Risk factors for retention of subretinal perfluorocarbon liquid in vitreoretinal surgery. Retina 2004;24:746-52.
46. Gass JD. Sympathetic ophthalmia following vitrectomy. Am J Ophthalmol 1982;93:552-8.
47. Pollack AL, McDonald HR, Ai E, et al. Sympathetic ophthalmia associated with pars plana vitrectomy without antecedent penetrating trauma. Retina 2001;21:146-54.
48. Liddy L, Stuart J. Sympathetic ophthalmia in
49. Lubin JR, Albert DM, Weinstein M. Sixty-five years of sympathetic ophthalmia. A clinicopathologic review of 105 cases (1913--1978). Ophthalmology 1980;87:109-21.
50. Marak GE, Jr. Sympathetic ophthalmia. Ophthalmology 1982;89:1291-2.
51. Fujii GY, De Juan E, Jr., Humayun MS, et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology 2002;109: 1807-12; discussion 13.
52. Kunimoto DY, Kaiser RS. Incidence of Endophthalmitis after 20- and 25-Gauge Vitrectomy. Ophthalmology 2007 Oct 2; [Epub ahead of print].
53. Lakhanpal RR, Humayun MS, de Juan E, Jr., et al. Outcomes of 140 consecutive cases of 25-gauge transconjunctival surgery for posterior segment disease. Ophthalmology 2005;112:817-24.
54. Gupta OP, Weichel ED, Regillo CD, et al. Postoperative complications associated with 25-ga. pars plana vitrectomy. Ophthalmic Surg Lasers Imaging 2007;38:270-5.
55. Shimada H, Nakashizuka H, Mori R, et al. 25-gauge scleral tunnel transconjunctival vitrectomy. Am J Ophthalmol 2006;142:871-3.
56. Lopez-Guajardo L, Pareja-Esteban J, Teus-Guezala MA. Oblique sclerotomy technique for prevention of incompetent wound closure in transconjunctival 25-gauge vitrectomy. Am J Ophthalmol 2006;141:1154-6.
57. McDonald HR, Schatz H, Allen AW, et al. Retinal toxicity secondary to intraocular gentamicin injection. Ophthalmology 1986;93:871-7.
58. Loewenstein A, Zemel E, Vered Y, et al. Retinal toxicity of gentamicin after subconjunctival injection performed adjacent to thinned sclera. Ophthalmology 2001; 108:759-64.