Irene A. Barbazetto, MD, New York City; Hideki Koizumi, MD, Kyoto, Japan; Lawrence A. Yannuzzi, MD, New York City
Myopia is one of the most common ocular conditions worldwide and has an estimated prevalence of 30,358,000 in the United States (25.4 percent of the population age 40 or older).1 While vision can be restored to 20/20 in most patients using glasses, contact lenses or refractive surgery, high or so called "pathologic myopia," defined by a refractive error >-6 D and/or an axial length of >25.5 mm, remains a significant risk for vision loss. According to a recent study from Japan, pathologic myopia was the third leading cause of bilateral vision loss and the leading cause of monocular vision loss in that population.2 The burden of myopia, however, is not limited to the Asian population; similar results were described by the Rotterdam Eye Study3 and in an Israeli population-based study, which named pathologic myopia as one of the top three causes of blindness.4
In spite of the public-health impact, only moderate progress has been made when it comes to the understanding of underlying pathological mechanisms and treatment strategies. The number of recent publications confirms this impression: 39 publications are listed in PubMed when searching for "pathologic myopia" in 2007 compared to 2,030 for "age-related macular degeneration" within the same year.
This review will try to summarize the most recent insights and developments with regard to the natural course, imaging and treatment strategies of retinal complications.
Visual Prognosis
While the visual prognosis in patients with subfoveal, choroidal neovascularization secondary to pathologic myopia is usually poor, significant variations in outcomes have been reported. While a 1984 study described myopic CNV as a "self-limited" disease,5 other reports were less optimistic in regard to functional preservation.6,7 Another group evaluated prognostic factors for good visual outcome over five years in 54 consecutive patients. They found that younger age, better baseline visual acuity and smaller CNV size correlated with a more favorable visual prognosis.8,9 However, similar to previous studies, this report found that 64.8 percent of their patients had a visual acuity of 20/200 or worse after five years.8 One of the additional sight-threatening events associated with CNV in myopia may be the development of choroidal atrophy around the fibrosing lesion. Another group found that age and CNV size related to the patient's age are the most significant risk factors.9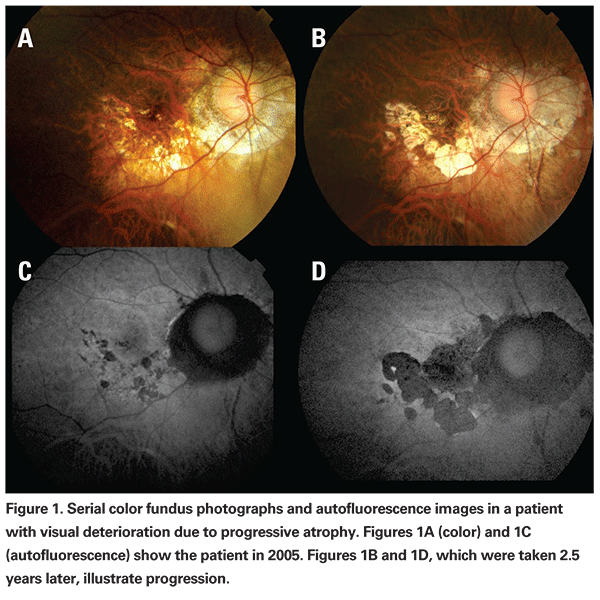
Visual impairment in pathologic myopia is not only caused by neovascular complications. Progressive atrophy (See Figure 1), foveal schisis, increased risk of retinal detachment, and glaucomatous optic neuropathy10 add to the general risk in patients with pathologic myopia. In a study that followed 552 highly myopic patients with and without signs of maculopathy over a 10-year period, researchers identified age as one of the most predictive factors for visual deterioration.11 Other aspects included the presence of macular changes, refractive status and axial length. While 92 percent of patients at age 40 to 49 had a final visual acuity of 20/40 or better, less than 40 percent of patients older than 60 years had a similar good outcome. Patients with signs of macular involvement (which included patients with lacquer cracks, choroidal atrophy, staphyloma or CNV) had more than a 50-percent chance of losing more than two lines of visual acuity over 10 years, compared to 4.3 percent for patients with no or only minimal macular involvement.
Schisis, Atrophy, Macular Holes
Recent developments in retinal imaging have contributed a better understanding of morphologic changes seen in pathologic myopia. Notably, optical coherence tomography has enabled detection of posterior fundus alterations associated with pathologic myopia, which are hard to see or differentiate biomicroscopically like the peripapillary retinal detachments, first described in 2003.12 While these lesions remain stable over many years and tend not to affect visual acuity, it is almost impossible to distinguish between neurosensory and RPE detachments without the help of OCT imaging in this pathology.
Myopic foveoschisis and foveal detachment with or without a macular hole, on the other hand, are characteristic findings that can threaten visual function (See Figure 2). Based on OCT imaging studies, these alterations are now thought to be caused by the combination of progressive elongation of the globe causing vitreoretinal interface abnormalities,13,14 as well as chorioretinal changes13,15 with vascular traction and retinal microfolds,16 which may precede the development of paravascular cysts, lamellar holes and macular schisis.17,18
Myopic foveoschisis without a macular hole is one of the common features and is observed in 9 to 34 percent of highly myopic eyes with a posterior staphyloma. However, the management of myopic foveoschisis is still in controversy. While some authors have described myopic foveoschisis as fairly stable with regard to visual acuity and retinal thickness,15 it can also present as a progressive disorder that can be additionally complicated by the presence of epiretinal membranes or a partially detached vitreous cortex.13,14 A study on the natural history demonstrated that approximately half of the patients with myopic macular schisis will develop a macular hole and/or foveal detachment within two or more years of diagnosis.19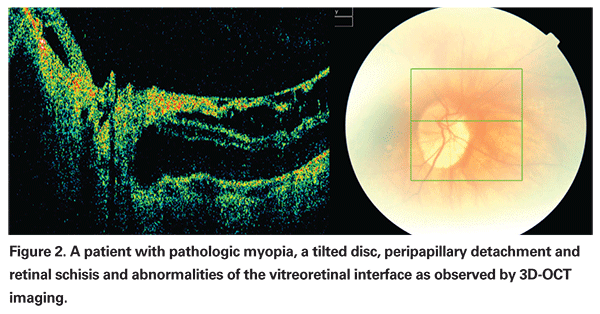
The prognostic value in regard to visual deterioration and the potential benefit of surgical intervention for myopic macular schisis still remains controversial and will need further investigation.13,20 Surgery may improve vision in a subset of patients with myopic foveal schisis, however, macular hole formation following surgery has been a feared complication. A retrospective study compared the natural course of myopic foveoschisis with patients who underwent surgery.14 The vision in the observed group remained stable for many years. The presence of premacular structures and abnormalities of the vitreoretinal interface, creating tangential surface forces, however, worsened the prognosis. For such cases, vitrectomy may be considered. But the presence of a foveal detachment in combination with vitreoretinal interface abnormalities alone was predictive of the development of macular holes with or without surgery. In another study, researchers proposed performing scleral buckling with a macular plombe in patients with macular schisis without hole in order to avoid the complications of vitrectomy and internal limiting membrane peeling.21 In their series, all patients had resolution of the macular detachment, and four out of six patients had visual improvement following the procedure.
Another group found that eyes with atrophic CNV were more likely to develop macular holes.17,22 In their series, the hole always occurred at the border of the former CNV and the surrounding choroidal atrophy.17 These retinal defects are difficult to detect by ophthalmoscopy alone. OCT imaging, especially with the recent generation of spectral domain devices, has proven to be a valuable addition in evaluating patients with impaired visual function as shown in Figure 3, where a hole was detected in a patient with extensive atrophic changes.
Treatment of CNV Secondary to PM
Five years ago, a review of the available study data in the Cochrane library failed to show a clear benefit of laser photocoagulation for any type of CNV secondary to PM.23 This was a rather expected result, since even patients with extrafoveal CNV potentially experience visual loss due to progressive enlargement of the laser scar over time.
To date, the VIP study (Verteporfin in Photodynamic Therapy) remains the largest controlled, double-blind study showing long-term benefits for treating subfoveal CNV secondary to PM. In this two-year study patients treated with verteporfin PDT (Visudyne) were significantly more likely to experience stabilization of visual acuity, or even improvement (12 percent), when compared to a placebo-treated control group.24 Long-term follow-up reports of patients treated with PDT, as well as recent pilot studies of patients treated for myopic CNV in Japan and China, confirmed lasting stabilization or even improvement in the majority of eyes. All of these studies used less frequent treatments than the VIP study.25,26 But atrophy, and even choroidal infarctions, have been reported more frequently in younger, myopic patients than in all other pathologies treated with PDT.27-31 These variations in response to treatment may explain why the one-year results of a study comparing PDT monotherapy with a combination treatment of PDT and intraocular triamcinolone injections, frequently used in AMD patients prior to the approval of anti-VEGF therapy, failed to show a significant difference in favor of combination therapy.32
Since the approval of ranibizumab (Lucentis) for subfoveal CNV in patients with AMD, treatment alternatives for several other types of CNV have been explored. It seems not surprising then that recent case reports and small case series treating pathologic myopia patients with anti-VEGF medications suggested that the exceptional results seen in patients with AMD can also be observed in patients with CNV secondary to pathologic myopia.33-38 However, larger, controlled clinical trials are not available for review at this time.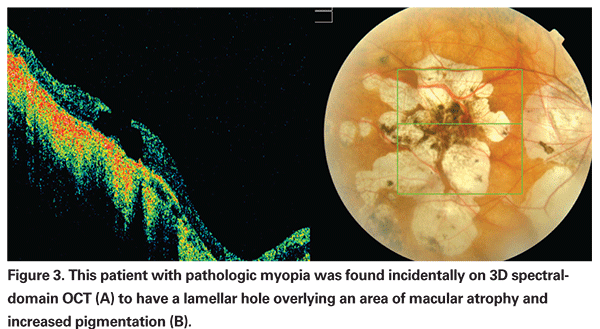
Advances in imaging technology such as OCTs and autofluorescence imaging have broadened our understanding of the mechanisms that are contributing to the visual deterioration in patients with pathologic myopia. In the future, these new insights together with advances in therapeutic interventions may help us to develop strategies to prevent vision loss in these patients.
The authors practice at Vitreous Retina and Macula Consultants of New York, and Dr. Koizumi is in the Department of Ophthalmology at Kyoto Prefectural University of Medicine,
1. Kempen JH, Mitchell P, Lee KE, Tielsch JM, et al. The prevalence of refractive errors among adults in the
2. Iwase A, Araie M, Tomidokoro A, Yamamoto T, et al. Prevalence and causes of low vision and blindness in a Japanese adult population: The Tajimi Study. Ophthalmology 2006;113:1354-1362.
3. Klaver CC, Wolfs RC, Vingerling JR, Hofman A, de Jong PT. Age-specific prevalence and causes of blindness and visual impairment in an older population: The Rotterdam Study. Arch Ophthalmol 1998;116:653-658.
4. Avisar R, Friling R, Snir M, Avisar I, Weinberger D. Estimation of prevalence and incidence rates and causes of blindness in Israel, 1998-2003. Isr Med Assoc J 2006;8:880-881.
5. Avila MP, Weiter JJ, Jalkh AE, Trempe CL et al. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology 1984;91:1573-1581.
6. Yoshida T, Ohno-Matsui K, Ohtake Y, Takashima T, et al. Long-term visual prognosis of choroidal neovascularization in high myopia: A comparison between age groups. Ophthalmology 2002;109:712-719.
7. Yoshida T, Ohno-Matsui K, Yasuzumi K, Kojima A, et al. Myopic choroidal neovascularization: A 10-year follow-up. Ophthalmology 2003;110:1297-1305.
8. Kojima A, Ohno-Matsui K, Teramukai S, Ishihara Y, et al. Estimation of visual outcome without treatment in patients with subfoveal choroidal neovascularization in pathologic myopia. Graefe's Arch Clin Exp Ophthalmol 2006;244:1474-1479.
9. Kojima A, Ohno-Matsui K, Teramukai S, Yoshida T, et al. Factors associated with the development of chorioretinal atrophy around choroidal neovascularization in pathologic myopia. Graefe's Arch Clin Exp Ophthalmol 2004;242:114-119.
10. Xu L, Wang Y, Wang S, Wang Y, Jonas JB. High myopia and glaucoma susceptibility the Beijing Eye Study. Ophthalmology 2007;114:216-220.
11. Shih YF, Ho TC, Hsiao CK, Lin LL. Visual outcomes for high myopic patients with or without myopic maculopathy: A 10-year follow-up study. Br J Ophthalmol 2006;90:546-550.
12. Freund KB, Ciardella AP, Yannuzzi LA, Pece A, et al. Peripapillary detachment in pathologic myopia. Arch Ophthalmol 2003;121:197-204.
13. Wu PC, Chen YJ, Chen YH, Chen CH, et al. Factors associated with foveoschisis and foveal detachment without macular hole in high myopia. Eye. 2007 Dec 7; [Epub ahead of print]
14. Gaucher D, Haouchine B, Tadayoni R, Massin P, et al. Long-term follow-up of high myopic foveoschisis: Natural course and surgical outcome. Am J Ophthalmol 2007;143:455-462.
15. Baba T, Ohno-Matsui K, Futagami S, Yoshida T, et al. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. Am J Ophthalmol 2003;135:338-342.
16. Ikuno Y, Gomi F, Tano Y. Potent retinal arteriolar traction as a possible cause of myopic foveoschisis. Am J Ophthalmol 2005;139:462-467.
17. Shimada N, Ohno-Matsui K, Yoshida T, Futagami S, et al. Development of macular hole and macular retinoschisis in eyes with myopic choroidal neovascularization. Am J Ophthalmol 2008;145:155-161.
18. Shimada N, Ohno-Matsui K, Yoshida T, Yasuzumi K, et al. Characteristics of peripapillary detachment in pathologic myopia. Arch Ophthalmol 2006;124:46-52.
19. Shimada N, Ohno-Matsui K, Baba T, Futagami S, et al. Natural course of macular retinoschisis in highly myopic eyes without macular hole or retinal detachment. Am J Ophthalmol 2006;142:497-500.
20. Ikuno Y, Sayanagi K, Ohji M, Kamei M, et al. Vitrectomy and internal limiting membrane peeling for myopic foveoschisis. Am J Ophthalmol 2004;137:719-724.
21. Baba T, Tanaka S, Maesawa A, Teramatsu T, et al. Scleral buckling with macular plombe for eyes with myopic macular retinoschisis and retinal detachment without macular hole. Am J Ophthalmol 2006;142: 483-487.
22. Shimada N, Ohno-Matsui K, Nishimuta A, Moriyama M, et al. Detection of paravascular lamellar holes and other paravascular abnormalities by optical coherence tomography in eyes with high myopia.
Ophthalmology 2008 Apr;115(4):708-17. Epub 2007 Aug 29.
23. Virgili G, Menchini F. Laser photocoagulation for choroidal neovascularisation in pathologic myopia. Cochrane database of systematic reviews (Online) 2005(4):CD004765.
24. Verteporfin in Photodynamic Therapy Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 1-year results of a randomized clinical trial--VIP report no. 1. Ophthalmology 2001;108:841-852.
25. Pece A, Vadala M, Isola V, Matranga D. Photodynamic therapy with verteporfin for juxtafoveal choroidal neovascularization in pathologic myopia: A long-term follow-up study. Am J Ophthalmol 2007; 143:449-454.
26. Hayashi K, Ohno-Matsui K, Teramukai S, Shimada N, et al. Photodynamic therapy with verteporfin for choroidal neovascularization of pathologic myopia in Japanese patients: Comparison with nontreated controls. Am J Ophthalmol 2008;145:518-526.
27. Parodi MB, Da Pozzo S, Ravalico G. Retinal pigment epithelium changes after photodynamic therapy for choroidal neovascularization in pathological myopia. Acta Ophthalmol Scand 2007;85:50-54.
28. Glacet-Bernard A, Coscas G, Soubrane G. Pigment epithelial changes in young women treated with photodynamic therapy and limited macular translocation for classic choroidal neovascularisation. Graefe's Arch Clin Exp Ophthalmol 2006;244:1373-1376.
29. Isola V, Pece A,
30. Ohno-Matsui K, Moriyama M, Hayashi K, Mochizuki M. Choroidal vein and artery occlusion following photodynamic therapy in eyes with pathologic myopia. Graefe's Arch Clin Exp Ophthalmol 2006;244: 1363-1366.
31. Hayashi K, Ohno-Matsui K, Teramukai S, Shimada N, et al. Photodynamic Therapy with Verteporfin for Choroidal Neovascularization of Pathologic Myopia in Japanese Patients: Comparison with Nontreated Controls. Am J Ophthalmol 2008;145:518-526.
32. Chan WM, Lai TY, Wong
33. Bennett MD, Yee W. Pegaptanib for myopic choroidal neovascularization in a young patient. Graefe's Arch Clin Exp Ophthalmol 2007;245:903-905.
34. Sakaguchi H, Ikuno Y, Gomi F, Kamei M, et al. Intravitreal injection of bevacizumab for choroidal neovascularisation associated with pathological myopia. Br J Ophthalmol 2007;91:161-165.
35. Chan WM, Lai TY, Liu DT, Lam DS. Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularization: Six-month results of a prospective pilot study. Ophthalmology 2007;114:2190-2196.
36. Laud K, Spaide RF, Freund KB, Slakter J, Klancnik Jr. JM. Treatment of choroidal neovascularization in pathologic myopia with intravitreal bevacizumab. Retina 2006;26:960-963.
37. Yamamoto I,
38. Mandal S, Venkatesh P, Sampangi R, Garg S. Intravitreal bevacizumab (Avastin) as primary treatment for myopic choroidal neovascularization. European J Ophthalmol 2007;17:620-626.