Eric B. Suhler, MD, MPH,
Emmett T. Cunningham Jr., MD, PhD, MPH,
Biologic response modifiers commonly referred to as biologics, is a term used to describe therapeutic proteins designed to block the activity of bioactive mediators of the immune response.most notably recombinant antibodies and antibody-derived proteins. Monoclonal antibodies (mAb), when used as medications, are given a generic name ending in "-mab" An antecedent "u"(-umab) indicates a human antibody; "xi"(-ximab) indicates a mixed human-murine (chimeric) antibody. Fusion proteins, which typically contain either receptor domains or cell surface markers, are given a generic name ending in "-cept."
Why Use Biologics in Uveitis?
Corticosteroids are the mainstay of treatment for immune-mediated diseases, including non-infectious uveitis and ocular inflammation, because they are inexpensive, potent and rapidly effective. However, corticosteroid-sparing immunosuppressive agents are required in a minority of patients to either control vision- or life-threatening autoimmune disease or to minimize toxicities associated with prolonged use of corticosteroids. Biologic therapy can be an alternative in patients with inadequate response to or tolerance of conventional immunotherapy.
The biologics reviewed here were initially developed to treat systemic inflammatory diseases or to prevent organ transplant rejection, but have been used off-label to treat uveitis or ocular inflammation. 1,2 Table 1 summarizes the Food and Drug Administration-approved systemic indications for many of the biologics. Ocular inflammation can occur with many of these diseases, including inflammatory bowel disease (IBD), ankylosing spondylitis (AS), psoriasis, Behcet"s disease (BD), juvenile idiopathic arthritis (JIA) and rheumatoid arthritis (RA).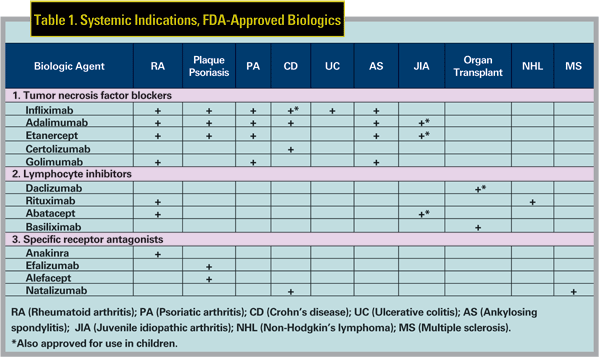
Which Biologic to Use in Uveitis?
Biologics are relatively new medications, and data on both efficacy and safety of these agents used to treat ocular conditions are limited. To date, none of the biologics discussed herein have been approved for the treatment of ocular inflammation. Off-label use of biologics to treat ocular immune-mediated diseases has appeared in a number of reports, all of them primarily uncontrolled small case series. The preponderance of literature describing biologic treatment of uveitis is with the tumor necrosis factor inhibitors (infliximab, etanercept and adalimumab), followed by the IL-2 receptor (IL2-R) blocker daclizumab.
All three commonly used TNF blockers were initially approved for the treatment of RA, and were subsequently approved for a number of other systemic indications (See Table 1).
Infliximab (Remicade, Figure 1) is a chimeric monoclonal antibody that binds both circulating and membrane-bound TNF-ƒ¿. Initial reports focused on the treatment of uveitis associated with BD and JIA. Infliximab has been reported to be a rapid and very effective therapy for the treatment of Behcet"s panuveitis,3-5 posterior uveitis, and retinal vasculitis.6 One group reported in a prospective study of 25 patients with Behcet"s uveitis that the majority (>90 percent) of patients experienced resolution of vitritis, retinitis, retinal vasculitis and cystoid macular edema within 28 days following initiation of infliximab therapy, and often as early as within one week.6 Efficacy of infliximab for JIA uveitis also has been demonstrated in several retrospective studies,7-10 with the majority of patients demonstrating rapid control of inflammation after the second infusion.7 Besides BD and JIA, infliximab has been reported anecdotally to be effective for the treatment of uveitis associated with multiple conditions, including IBD,11-13 AS,14 psoriasis,14 sarcoidosis,5,12,15-17 Vogt- Koyanagi-Harada disease,12 and Takayasu disease.18 It may also be effective to treat birdshot retinochoroidopathy,5,12,19 recalcitrant uveitic cystoid macular edema,20 pars planitis,5,8 multifocal choroiditis,5 HLA-B27-related anterior uveitis,14,21,22 and idiopathic uveitis.5,12,19 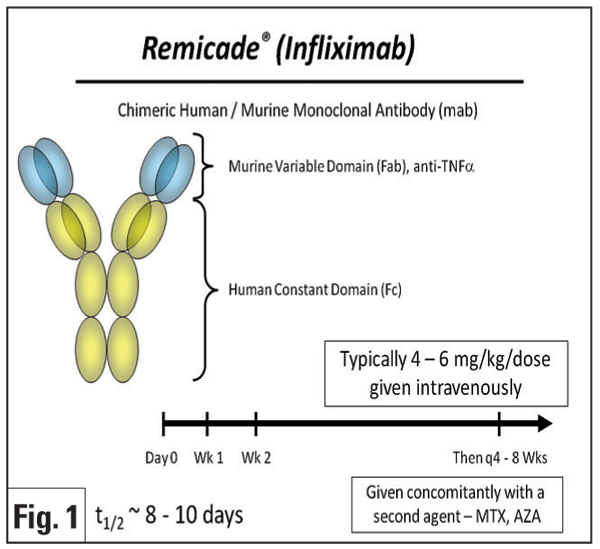
Etanercept (Enbrel, Figure 2) is a fusion protein of a human Fc molecule and two p75 TNF receptors that binds free TNF-á and -â. A prospective pilot study in 10 patients with JIA uveitis showed no significant difference in control of anterior chamber inflammation between those who received etanercept and controls.23 Other research reported that etanercept was not superior to placebo in preventing uveitic relapses in previously controlled patients attempting to taper methotrexate.24 In one prospective study, favorable outcomes at three months were seen in 63 percent of pediatric eyes treated with etanercept.25 Although a subsequent, larger, prospective, open-label, multicenter study with longer follow-up (median of 13 months) showed that patients with JIA may initially respond to etanercept, only approximately half of responders had sustained improvement at one year. The same study indicated that etanercept was associated with a wide array of severe side effects that disappeared after discontinuation of treatment.26 Based on the existing non-controlled data, infliximab generally is believed to be more effective than etanercept for JIA10 and various uveitides.27 However, it is important to note that no head-to-head study of the effectiveness or safety of the TNF-blockers has been prospectively performed, and infliximab has also been reported to have significant toxicity.5 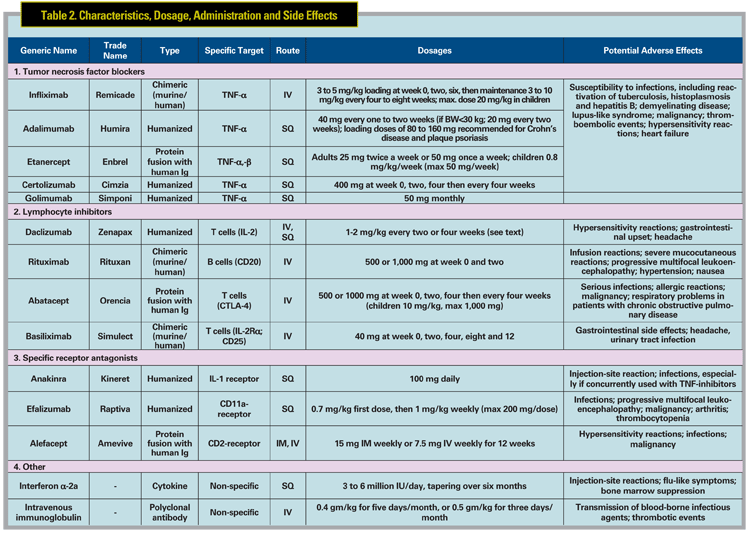
Adalimumab (Humira, Figure 3) is a fully human monoclonal antibody against TNF-á. Due to its promising results and subcutaneous route of administration, experience in both rheumatologic and ocular indications is growing. Several clinical studies have shown its potential for juvenile uveitis (mainly JIA), with data in adults relatively lacking. A prospective, non-comparative, nonrandomized study in 14 children with recalcitrant uveitis (nine JIA and five idiopathic) demonstrated improvement of anterior chamber inflammation in 93 percent of cases.28 A similar retrospective study for JIA demonstrated control of uveitis and arthritis activity in more than 80 percent of cases, with median response time of three weeks for arthritis and six weeks for uveitis.29 A more recent retrospective study for JIA uveitis revealed that adalimumab effectively controlled inflammation in approximately one-third of patients refractory to previous treatment with infliximab or etanercept.30 A prospective open-label study of adalimumab in 19 adults with various uveitides reported control of inflammation in 63 percent of patients and complete resolution of CME in 55 percent of eyes at one year of therapy.31 The literature for treatment of BD uveitis is less robust than that published for infliximab; however, one group showed that adalimumab could be an effective alternative for treatment of BD-associated panuveitis in patients already well-controlled with infliximab wishing to avoid intravenous infusions.32 Adalimumab also has been shown to reduce anterior uveitis flares in patients with AS.33 A Phase II, multicenter, prospective clinical trial of adalimumab for the treatment of refractory non-infectious uveitis in adults is ongoing, and should further inform the use of adalimumab for refractory uveitis.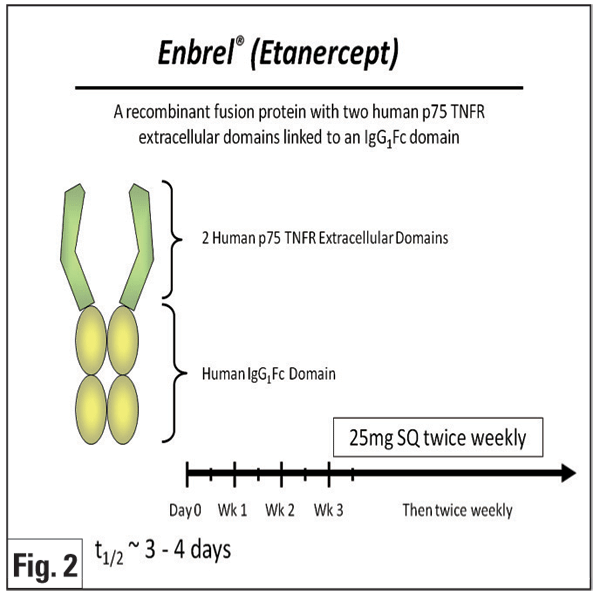
Daclizumab (Zenapax) is a monoclonal antibody that binds the IL2-R on T lymphocytes, preventing their IL2- dependent activation. A Phase I/II openlabel study of intravenous daclizumab for the treatment of non-infectious intermediate, posterior or panuveitis demonstrated improvement of inflammation and visual acuity at one year in eight of 10 patients,34 which was maintained after four years of treatment.35 Increased uveitic recurrences were observed with reduction to six-weekly infusions compared to four-weekly infusions. After four years of intravenous infusions, some patients were transitioned to subcutaneous daclizumab therapy, with most patients retaining good response.35 Ten of 15 patients in a subsequent study achieved at least a 50-percent reduction of concurrent immunosuppression with retained visual acuity, inflammatory control, and no significant side effects.36 Daclizumab has been demonstrated effective for refractory birdshot retinochoroidopathy in one study, with seven of eight patients achieving complete resolution of vitreous inflammation and stabilization or improvement of visual acuity.37 However, daclizumab failed to demonstrate effectiveness in BD-associated uveitis in a randomized trial.38 A trial of the chimeric IL-2 receptor basiliximab was recently proposed but withdrawn, pending further studies in non-ophthalmic indications.
Other biologics of interest under current study include efalizumab and rituximab. Efalizumab blocks the pan-leukocyte surface marker CD11a and is under current study for non-infectious uveitis with macular edema. The B-cell antagonist rituximab is currently under study for treatment of scleritis and orbital inflammation and has reported effectiveness for uveitis in one case report.39 Isolated reports also have suggested benefit for the IL-1 blocker anakinra40,41 and the T-cell costimulation inhibitor abatacept.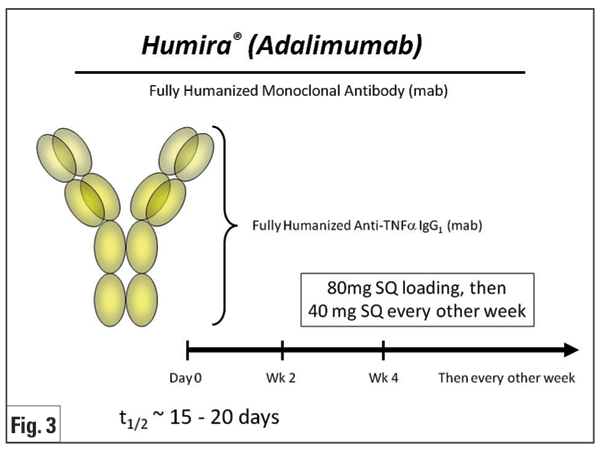
When to Use Biologics in Uveitis?
Biologics are useful when standard immunosuppression has failed or has been poorly tolerated, or to treat patients with concomitant ocular and systemic inflammation that might benefit from these medications. Given their cost as well as the limited long-term safety data, we do not routinely recommend biologics as first-line therapy. Situational exceptions for earlier use may be made for BD-associated uveitis, where infliximab has been demonstrated to be rapidly effective. Consideration may also be given to earlier use of biologics in patients with systemic disease, such as rheumatoid arthritis, AS and JIA, particularly when institution of such therapy will provide treatment benefit for both systemic and ocular inflammation. Excepting these situations, we generally reserve biologics for cases where "standard" immunosuppression has either failed or been poorly tolerated due to side effects, or in diseases known to be associated with a high risk of vision loss or irreversible complications related to uncontrolled inflammation.
Special Considerations
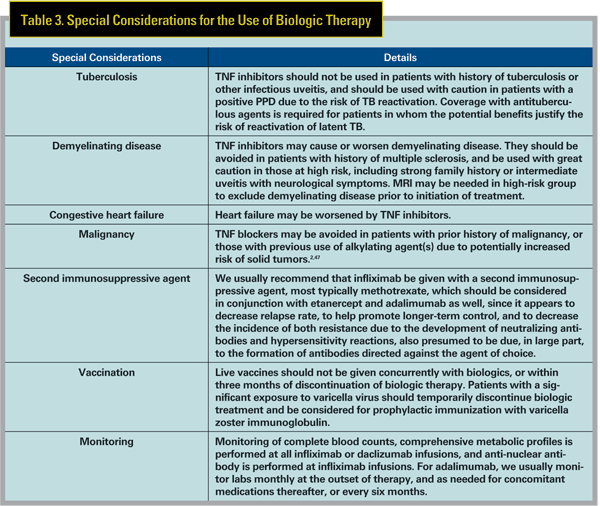
In general, antibodies created fully or in part from nonhuman DNA (e.g., infliximab) are more likely to cause hypersensitivity reactions and to result in the creation of neutralizing antibodies when compared to fully humanized (e.g., adalimumab) antibodies. This is why infliximab is always given with a second immunosuppresive agent, typically methotrexate, to suppress the formation of such unwanted antibodies, whereas use of a second agent in association with adalimumab is optional. Infliximab-associated infusion reactions can be severe, but are usually mild and treatable with antihistamines and/or corticosteroids. Etanercept and adalimumab are administered by subcutaneous injection and may cause self-limited inflammation at the injection site.1 Other biologics can also cause hypersensitivity reactions, headache, flu-like symptoms, gastrointestinal adverse effects, and they also may increase risk of infection and malignancy. Special considerations which need to be focused in each patient receiving biologic therapy are listed in Table 3.
Children, Pregnancy and Lactation
Biologics that are FDA-approved in systemic pediatric diseases include infliximab (Crohn"s disease; >6 years old), etanercept (JIA; >2 years old), adalimumab (JIA; >4 years old), daclizumab (organ transplant; >11 months old) and abatacept (JIA; >6 years old). Rituximab and anakinra have been used off-label in children, but are not FDA-approved in children. As noted above, off-label use of biologics has been extensively reported in children with uveitis, and has been useful in our hands, although higher doses may be required.
The FDA pharmaceutical categories for biologics and other immunosuppressives are reviewed in Table 4. In general, biologics should not be used in pregnant or lactating women unless the potential benefit justifies the risk to the fetus/ infant, which is not well-qualified by longterm study. Thorough discussion with the patient of the risks and benefits, both ocular and obstetric, is required, and consultation with an obstetrician experienced in high-risk pregnancies is invaluable in these situations.
Other Biologics
Interferons, including IFN ƒ¿-2a and ƒÀ-1a have been reported to be useful in the treatment of refractory ocular inflammation and associated macular edema,42 particularly in patients with BD43 and multiple sclerosis.44 However, relapse is frequently seen after discontinuation of therapy, and potentially serious complications may occur. Multiple cases of possible IFN-induced sarcoidosis with45 or without46 uveitis have been reported.
Intravenous immunoglobulin (IVIg) has been used successfully for treatment of refractory uveitis.47,48 The mechanism of action remains unknown. IVIg is not associated with systemic immunosuppression, reducing the risk of opportunistic infection. However, IVIg is extremely expensive and can cause severe side effects including thromboembolism, aseptic meningitis and bloodborne infections, and is usually reserved as a treatment of last resort.
Vascular endothelial growth factor inhibitors, including bevacizumab and ranibizumab, widely used to treat age-related macular degeneration and other retinal vascular diseases, have reported benefit in reducing uveitic CME in selected patients with otherwise well-controlled uveitis, at least transiently.49,50 Preliminary safety studies to evaluate toxicity of intravitreal injection of TNF inhibitors, including infliximab,51 etanercept52 and adalimumab,53 have been performed in rabbits with experimental uveitis, with promising results.
Biologics are potent new agents approved for a number of rheumatologic conditions. Use in uveitis is largely anecdotal and suggests that some, but not all, patients who are refractory to more traditional immunosuppressive therapies may benefit from biologics. We are currently unable to identify a priori which patients might benefit from treatment with biologics, but increasing evidence suggests particularly promising efficacy in patients with Behcet"s disease and JIA. Side effects are not uncommon and can be quite serious, so patients should be apprised of both the risks as well as the potential benefits of these agents. For ophthalmologists who are inexperienced in the use of biologics, we recommend working closely with an experienced uveitis specialist and a rheumatologist. Due to cost and limited long-term safety data, we continue to reserve biologics for use in patients with uveitis refractory to more traditional immunosuppressive therapies.
Dr. Pasadhika is a retinal surgeon and chief of the Uveitis Service at Chulalongkorn University in Bangkok, Thailand. Dr. Suhler is chief of ophthalmology at Portland Veterans Administration Medical Center, an associate professor of ophthalmology at Oregon Health & Science University, and codirector of the Uveitis Clinic at the Casey Eye Institute/Oregon Health & Science University. Dr. Cunningham is director of the Uveitis Service at CPMC, an adjunct clinical professor of ophthalmology at Stanford University School of Medicine, and in private practice at West Coast Retina Medical Group in San Francisco. Correspondence should be addressed to Dr. Suhler at suhlere@ ohsu.edu.
1. Lim L, Suhler EB, Smith JR. Biologic therapies for inflammatory eye disease. Clin Experiment Ophthalmol 2006;34:365-74.
2. Imrie FR, Dick AD. Biologics in the treatment of uveitis. Curr Opin Ophthalmol 2007;18:481-6.
3. Sfikakis PP, Theodossiadis PG, Katsiari CG, Kaklamanis P, Markomichelakis NN. Effect of infliximab on sight-threatening panuveitis in Behçet's disease. Lancet 2001;358:295-6.
4. Ohno S, Nakamura S, Hori S, et al. Efficacy, safety and pharmacokinetics of multiple administration of infliximab in Behçet's disease with refractory uveoretinitis. J Rheumatol 2004;31:1362-8.
5. Suhler EB, Smith JR, Wertheim MS, et al. A prospective trial of infliximab therapy for refractory uveitis: preliminary safety and efficacy outcomes. Arch Ophthalmol 2005;123:903-12.
6. Sfikakis PP, Kaklamanis PH, Elezoglou A, et al. Infliximab for recurrent, sight-threatening ocular inflammation in Adamantiades-Behçet's disease. Ann Intern Med 2004;140:404-6.
7. Kahn P, Weiss M, Imundo LF, Levy DM. Favorable response to high-dose infliximab for refractory childhood uveitis. Ophthalmology 2006;113:860-4 e2.
8. Rajaraman RT, Kimura Y, Li S, Haines K, Chu DS. Ret-rospective case review of pediatric patients with uveitis treated with infliximab. Ophthalmology 2006;113:308-14.
9. Richards JC, Tay-Kearney ML, Murray K, Manners P. Infliximab for juvenile idiopathic arthritis- associated uveitis. Clin Experiment Ophthalmol 2005;33:461-8.
10. Tynjala P, Lindahl P, Honkanen V, Lahdenne P, Kotaniemi K. Infliximab and etanercept in the treatment of chronic uveitis associated with refractory juvenile idiopathic arthritis. Ann Rheum Dis 2007;66:548-50.
11. Fries W, Giofre MR, Catanoso M, Lo Gullo R. Treatment of acute uveitis associated with Crohn's disease and sacroileitis with infliximab. Am J Gastroenterol 2002;97:499-500.
12. Baughman RP, Bradley DA, Lower EE. Infliximab in chronic ocular inflammation. Int J Clin Pharmacol Ther 2005;43:7-11.
13. Rispo A, Scarpa R, Di Girolamo E, et al. Infliximab in the treatment of extra-intestinal manifestations of Crohn's disease. Scand J Rheumatol 2005;34:387-91.
14. Bodaghi B, Bui Quoc E, Wechsler B, et al. Therapeutic use of infliximab in sight threatening uveitis: retrospective analysis of efficacy, safety, and limiting factors. Ann Rheum Dis 2005;64:962-4.
15. Pritchard C, Nadarajah K. Tumour necrosis factor alpha inhibitor treatment for sarcoidosis refractory to conventional treatments: a report of five patients. Ann Rheum Dis 2004;63:318-20.
16. Doty JD, Mazur JE, Judson MA. Treatment of sarcoidosis with infliximab. Chest 2005;127:1064-71.
17. Benitez-del-Castillo JM, Martinez-de-la-Casa JM, Pato-Cour E, et al. Long-term treatment of refractory posterior uveitis with anti-TNFalpha (infliximab). Eye 2005;19:841-5.
18. Jolly M, Curran JJ. Infliximab-responsive uveitis and vasculitis in a patient with Takayasu arteritis. J Clin Rheumatol 2005;11:213-5.
19. Lindstedt EW, Baarsma GS, Kuijpers RW, van Hagen PM. Anti-TNF-alpha therapy for sight threatening uveitis. Br J Ophthalmol 2005;89:533-6.
20. Markomichelakis NN, Theodossiadis PG, Pantelia E, Papaefthimiou S, Theodossiadis GP, Sfikakis PP. Infliximab for chronic cystoid macular edema associated with uveitis. Am J Ophthalmol 2004;138:648-50.
21. El-Shabrawi Y, Hermann J. Anti-tumor necrosis factor-alpha therapy with infliximab as an alternative to corticosteroids in the treatment of human leukocyte antigen B27-associated acute anterior uveitis. Ophthalmology 2002;109:2342-6.
22. Kruithof E, Kestelyn P, Elewaut C, et al. Successful use of infliximab in a patient with treatment resistant spondyloarthropathy related uveitis. Ann Rheum Dis 2002;61:470.
23. Smith JA, Thompson DJ, Whitcup SM, et al. A randomized, placebo-controlled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum 2005;53:18-23.
24. Foster CS, Tufail F, Waheed NK, et al. Efficacy of etanercept in preventing relapse of uveitis controlled by methotrexate. Arch Ophthalmol 2003;121:437-40.
25. Reiff A, Takei S, Sadeghi S, et al. Etanercept therapy in children with treatment-resistant uveitis. Arthritis Rheum 2001;44:1411-5.
26. Quartier P, Taupin P, Bourdeaut F, et al. Efficacy of etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthritis Rheum 2003;48:1093-101.
27. Galor A, Perez VL, Hammel JP, Lowder CY. Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology 2006;113:2317-23.
28. Vazquez-Cobian LB, Flynn T, Lehman TJ. Adalimumab therapy for childhood uveitis. J Pediatr 2006;149:572-5.
29. Biester S, Deuter C, Michels H, et al. Adalimumab in the therapy of uveitis in childhood. Br J Ophthalmol 2007;91:319-24.
30. Tynjala P, Kotaniemi K, Lindahl P, et al. Adalimumab in juvenile idiopathic arthritisassociated chronic anterior uveitis. Rheumatology (Oxford) 2008;47:339-44.
31. Diaz-Llopis M, Garcia-Delpech S, Salom D, et al. Adalimumab therapy for refractory uveitis: a pilot study. J Ocul Pharmacol Ther 2008;24:351-61.
32. Mushtaq B, Saeed T, Situnayake RD, Murray PI. Adalimumab for sight-threatening uveitis in Behcet's disease. Eye 2007;21:824-5.
33. Rudwaleit M, Rodevand E, Holck P, et al. Adalimumab effectively reduces the rate of anterior uveitis flares in patients with active ankylosing spondylitis: results of a prospective open-label study. Ann Rheum Dis 2009;68:696-701.
34. Nussenblatt RB, Fortin E, Schiffman R, et al. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proc Natl Acad Sci USA 1999;96:7462-6.
35. Nussenblatt RB, Thompson DJ, Li Z, et al. Humanized anti-interleukin-2 (IL-2) receptor alpha therapy: Long-term results in uveitis patients and preliminary safety and activity data for establishing parameters for subcutaneous administration. J Autoimmun 2003;21:283-93.
36. Nussenblatt RB, Peterson JS, Foster CS, et al. Initial evaluation of subcutaneous daclizumab treatments for noninfectious uveitis: a multicenter noncomparative in-terventional case series. Ophthalmology 2005;112:764-70.
37. Sobrin L, Huang JJ, Christen W, Kafkala C, et al. Da- clizumab for treatment of birdshot chorioretinopathy. Arch Ophthalmol 2008;126:186-91.
38. Buggage RR, Levy-Clarke G, Sen HN, et al. A double-masked, randomized study to investigate the safety and efficacy of daclizumab to treat the ocular complications related to Behçet's disease. Ocul Immunol Inflamm 2007;15:63-70.
39. Tappeiner C, Heinz C, Specker C, Heiligenhaus A. Rituximab as a treatment option for refractory endogenous anterior uveitis. Ophthalmic Res 2007;39:184-6.
40. Botsios C, Sfriso P, Furlan A, Punzi L, Dinarello CA. Resistant Behçet's disease responsive to anakinra. Ann Intern Med 2008;149:284-6.
41. Teoh SC, Sharma S, Hogan A, Lee R, Ramanan AV, Dick AD. Tailoring biological treatment: Anakinra treatment of posterior uveitis associated with the CINCA syndrome. Br J Ophthalmol 2007;91:263-4.
42. Pasadhika S, Smith J. Treatment of uveitic cystoid macular edema: an overview and update. Ophthalmology International 2008:97-103.
43. Gueudry J, Wechsler B, Terrada C, et al. Longterm efficacy and safety of low-dose interferon alpha2a therapy in severe uveitis associated with Behçet's disease. Am J Ophthalmol 2008;146:837-44.
44. Becker MD, Heiligenhaus A, Hudde T, et al. Interferon as a treatment for uveitis associated with multiple sclerosis. Br J Ophthalmol 2005;89:1254-7.
45. Doycheva D, Deuter C, Stuebiger N, Zierhut M. Interferon-alpha-associated presumed ocular sarcoidosis. Graefe's Arch Clin Exp Ophthalmol 2008.
46. Alazemi S, Campos MA. Interferon-induced sarcoidosis. Int J Clin Pract 2006;60:201-11.
47. Rosenbaum JT, George RK, Gordon C. The treatment of refractory uveitis with intravenous immunoglobulin. Am J Ophthalmol 1999;127:545- 9.
48. Onal S, Foster CS, Ahmed AR. Efficacy of intravenous immunoglobulin treatment in refractory uveitis. Ocul Immunol Inflamm 2006;14:367-74.
49. Cordero Coma M, Sobrin L, Onal S, Christen W, Foster CS. Intravitreal bevacizumab for treatment of uveitic macular edema. Ophthalmology 2007;114:1574-1579.
50. Mackensen F, Heinz C, Becker MD, Heiligenhaus A. Intravitreal bevacizumab (avastin) as a treatment for refractory macular edema in patients with uveitis: A pilot study. Retina 2008;28:41-5.
51. Theodossiadis PG, Liarakos VS, Sfikakis PP, et al. Intravitreal administration of the anti-TNF monoclonal antibody Infliximab in the rabbit. Graefe's Arch Clin Exp Ophthalmol 2008.
52. Fauser S, Kalbacher H, Alteheld N, Koizumi K, Krohne TU, Joussen AM. Pharmacokinetics and safety of intravitreally delivered etanercept. Graefe's Arch Clin Exp Ophthalmol 2004;242:582-6.
53. Manzano RP, Peyman GA, Carvounis PE, et al. Ocular toxicity of intravitreous adalimumab (Humira) in the rabbit. Graefe's Arch Clin Exp Ophthalmol 2008;246:907-11.



