 |
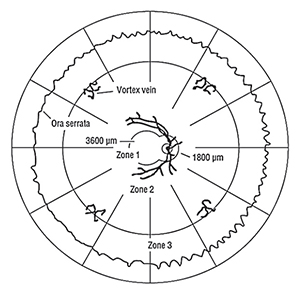
| Figure 1. Retinal Zones. One disc diameter is approximately 1,800 µm.1-3 |
CMVR has been associated with the human immunodeficiency virus infection since the 1980s.16 CMVR occurs in 15 to 40 percent of acquired immune deficiency syndrome patients.5 The advent of highly active antiretroviral therapy (HAART) in the 1990s has reduced the incidence of CMVR by 90 percent in the United States.9,17 HAART has reduced the rate of CMVR-associated rhegmatogenous retinal detachments by 60 percent.5
CMVR typically occurs when CD4+ T-cell counts fall below 50 cells/µl, and rarely occurs at counts above 100 cells/µl.2,9 Immune recovery uveitis refers to intraocular inflammation that occurs in HIV-positive patients with prior, inactive CMVR, in whom the CD4+ counts rise above 100 cells/µl. IRU occurs in up to 63 percent of patients with prior CMVR.18
Uveitic manifestations of IRU include vitritis; vitreomacular traction; cystoid macular edema; epiretinal membrane; papillitis; neovascularization; proliferative vitreoretinopathy; and RRD.17,18 The vitritis and CME of IRU can be treated with periocular or intravitreal triamcinolone, but with caution, so as to avoid recurrence.2 Systemic CMV manifestations include encephalitis; esophagitis; colitis; pneumonitis; hepatitis; leukopenia; and arthralgia. Systemic CMV disease occurs in about 25 percent of patients with CMVR.5
CMVR can also occur in non-HIV patients, but is much less common. In a recent comprehensive review of 178 HIV-negative patients with CMVR, factors that contributed to relative immunosuppression included age over 60 years (33.1 percent); leukemia (19.7 percent); systemic autoimmune disease (19.1 percent); organ transplantation (15.2 percent); lymphoma (7.9 percent); diabetes mellitus (6.1 percent); and multiple myeloma (1.7 percent).19 CMVR has been described in nine patients with Good syndrome.19,20 Among 105 HIV-negative CMVR patients on immunosuppressives, CMVR was found in patients on corticosteroids (65.7 percent); cyclophosphamide (31.4 percent); azathioprine (16.2 percent); methotrexate (14.3 percent); cyclosporine (12.4 percent); tacrolimus (10.5 percent); mycophenolate (7.6 percent); and fludarabine (6.7 percent).19 CMVR can also occur following periocular or intraocular corticosteroid injection.4,13,19-22 IRU can also occur in HIV-negative patients with prior CMVR, but
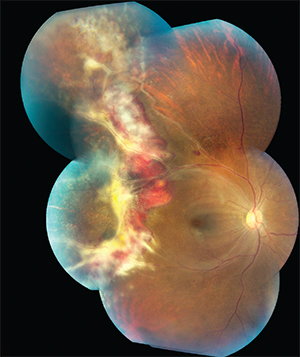 |
| Figure 2: Color fundus photograph of the right eye of an HIV-positive 35-year-old female. The hemorrhage and vascular sheathing suggests fulminant CMVR. Encroachment of the CMVR into the superotemporal macular area suggests Zone 1 involvement. The border opacification at 9 o’clock matches the standard peripheral photograph of severe (4+) border opacification; this is active retinitis. The CMVR involves approximately 25 percent of the retina surface of the right eye. This patient died of AIDS soon after this photograph was taken. |
Many patients with CMVR are asymptomatic, as the retinitis often begins in the peripheral retina. Screening for CMVR is indicated for all HIV-positive patients with a CD4+ count less than 100 cells/µl.2,5
Classical CMVR is a slowly progressive necrotizing retinitis that may affect the posterior pole and periphery, and may be unilateral or bilateral. White intraretinal infiltrates are often seen along the vascular arcades and are associated with necrotic areas and retinal hemorrhages. CMVR progression leads to retinal atrophy. Anterior segment inflammation with or without keratic precipitates is often present. Optic atrophy is a common late manifestation.2,5
CMVR can be classified into indolent or fulminant CMVR. Indolent CMVR consists of granular retinal opacification with visible choroidal details with no hemorrhage and no vascular sheathing. Fulminant (edematous) CMVR consists of dense confluent retinal opacification of necrotizing retinitis and hemorrhages with no atrophic areas.3
CMVR assessment should include grading of the area of retina involvement into three zones (See Figure 1). Zone 1 is the area 3,000 µm from the fovea and 1,500 µm from the optic disc. Zone 2 is from Zone 1 to the vortex veins. Zone 3 is the remaining retina up to the ora serrata.1,23 In HIV-positive patients, the majority of CMVR is peripheral (i.e., Zone 1-sparing; 61 to 71 percent of eyes) with only a minority involving Zone 1 (i.e., 29 to 39 percent of eyes).17,24 CMVR assessment should also include estimation of the percentage of the total retinal surface area affected by the CMVR (e.g., <10 percent, 10 to 24 percent, 25 to 50 percent, >50 percent) since this has implications regarding the risk of retinal detachment.24
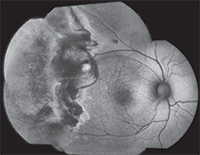 |
| Figure 3. Fundus autofluorescence photograph of the same patient from figure 2. Intense, confluent hyperautofluorescence temporal to the macula corresponds to the advancing, active edge of retinitis. Mottled hyper- and hypoautofluorescence peripheral to the area of active retinitis corresponds to a large area of previously infected, but not necrotic, atrophic retina.8 |
CMVR borders can be graded based on photographs. Mild (1+) is where the CMVR border is faint and does not obscure the choroid. Moderate (2+) is where the CMVR border consists of isolated areas of denser retinal whitening which partially obscures the choroid. Marked (3+) is confluent retinal whitening, partially obscuring the choroid. Severe (4+) is where the border opacification is so dense that the choroid cannot be seen, indicating active retinitis.3,7 Screening has revealed that 42.9 percent of CMVR eyes have 1+ opacification; 28.6 percent have 2+ opacification; 4.7 percent have 3+ opacification; and 23.8 percent have 4+ opacification.24 Figures 2 and 3 show color and fundus autofluorescence montages of active CMVR in an HIV-positive patient.
Differential Diagnosis
The differential diagnoses for CMVR is shown in Table 1.5 Most of these conditions can be differentiated from CMVR clinically, but retinitides mediated by HHV family members (e.g., CMV, herpes simplex virus and varicella zoster virus) may be more difficult to distinguish clinically.5,6
| Table 1. Differential Diagnoses of Cytomegalovirus Retinitis | |
| Infective causes | Non-infective causes |
| Acute retinal necrosis (ARN) | Behçet’s disease |
| Progressive (outer) retinal necrosis (PORN) | Primary vitreoretinal lymphoma (PVRL) |
| Herpes simplex virus retinitis | |
| oxoplasmosis | |
| Candida infection | |
| Syphilis | |
| Subacute sclerosing panencephalitis (SSPE) | |
An aqueous tap for polymerase chain reaction-based testing can distinguish between CMV, HSV and VZV infection. An anterior chamber tap typically provides 50 to 150 µl of fluid. A vitreous tap provides 300 µl of fluid. Among 178 HIV-negative CMVR eyes, 71.8 percent had their diagnosis of CMVR confirmed by PCR.19 PCR for toxoplasmosis could also be requested if there is a concern.25 It should be noted that the sensitivity of PCR-based testing falls if the sample is obtained following the initiation of anti-viral therapy.4
Serial fundus photography and wide-angle fluorescein angiography can be helpful in the management of patients with CMVR. Wide-angle imaging, in particular, can demonstrate peripheral ischemia, which may require pan-retinal photocoagulation.26 In HIV-negative CMVR patients, an occlusive vasculopathy was reported in 47 of 199 eyes—or nearly one in four cases.19
RRD occurs in about 20 to 30 percent of HIV-infected CMVR patients, and occurs most often in areas of prior retinal necrosis.2,5,27 The risk of RRD increases as the amount of peripheral retina affected by the CMVR increases.2 For post-CMVR RRD, a retina reattachment rate of 86 percent can be achieved with a 360 degree encircling band, vitrectomy, endolaser and silicone oil injection.5,28
| Table 2. Systemic Antivirals | |||
| Drug | Route | Dosage & Timing | Side Effects (by %) and Comments |
| Valganciclovir | Peroral | 900 mg b.i.d. for three weeks (induction); 900 mg daily (maintenance) | Diarrhea (38%), nausea (23), neutropenia (10), anemia (12), thrombocytopenia (2) |
| Ganciclovir | Intravenous | 5 mg/kg q12h for two to three weeks (induction); 5 mg/kg/day (maintenance) | Granulocytopenia (33), neurologic dysfunction, abnormal liver function, thrombocytopenia. |
| Foscarnet | Intravenous | 90 mg/kg b.i.d. for two to three weeks (induction); 90 mg/kg daily (maintenance) | Nephrotoxicity, neutropenia, anemia, hypocalcemia, Mg2+, PO4- abnormalities. Consider saline loading. |
| Cidofovir | Intravenous | 5 mg/kg once a week for two to three weeks (induction); 3 to 5 mg/kg alternate weeks (maintenance) | Iritis (50), ocular hypotony. Probenecid and IV hydration reduces nephrotoxicity. |
Treatment of CMVR
Treatment options for CMVR vary. Systemic options are shown in Table 2. Local options are shown in Table 3.2,5,29
The gold standard for the treatment of CMVR, in both HIV-positive and HIV-negative patients, is systemic antiviral therapy.15,17 Systemic therapy is generally preferred both because it reduces overall morbidity and because it generally works, with nearly three-quarters of patients achieving a post-treatment vision of 20/40 or better.17 First-line treatment for both HIV-positive and HIV-negative patients with CMVR includes either intravenous ganciclovir or oral valganciclovir.5,15
If an HIV-positive patient’s CD4+ count cannot be elevated with HAART, systemic antiviral therapy may need to become lifelong. This typically requires placement of a permanent indwelling venous catheter. If the CD4+ count can be kept above 100 cells/µl for three to six months, anti-CMV treatment typically can be stopped. Oral valganciclovir is as effective as IV ganciclovir, and allows patients to be maintained without long-term intravenous access.5
| Table 3. Local Antivirals | |||
| Drug | Route | Dosage & Timing | Comments |
| Ganciclovir | Intravitreal | 2 mg twice a week (induction*); 2 mg weekly (maintenance)15, 30 | 5 mg weekly, high-dose possible.31 |
| Foscarnet | Intravitreal | 2.4 mg, one to two times a week (induction); 2.4 mg weekly (maintenance)32 | Costly.32 |
| Cidofovir | Intravitreal | 20 µg every five to six weeks32 | Long-acting. Give oral probenecid 2 g two hours before injection, and 1 g two hours and eight hours after injection. Hypotony and iritis risks are lower with a 10-µg dose. |
| Fomivirsen | Intravitreal | Two doses 330 µg (0.05 ml), two weeks apart (induction); 330 µg monthly (maintenance) | No longer available in the United States. Reports of peripheral retinal toxicity and inflammation. |
| Ganciclovir (Vitrasert) | Sustained-release implant | 1 µg per hour for eight months | No longer available.5, 29 |
CMV resistance to ganciclovir can occur with long-term antiviral therapy.5,25 When ganciclovir resistance does occur, treatment options include the use of systemic foscarnet, or use of adjunctive high-dose intravitreal antiviral foscarnet or ganciclovir.31,33 Combination intravenous ganciclovir/foscarnet or oral valganciclovir plus intravenous foscarnet is more effective than monotherapy in resistant CMVR.5 While a 2-mg intravitreal injection of ganciclovir maintains therapeutic vitreous levels for up to seven days,34,15 use of intravitreal injections alone has been associated with worse visual acuity outcomes—at least in HIV-positive patients.2,35
As many CMVR patients are initially asymptomatic, screening for CMVR is recommended. CMVR progresses approximately 250 µm per week.2,37 Currently no laboratory marker exists that predicts the occurrence of CMVR.5 Clinical examination by a fellowship-trained retina specialist diagnosed CMVR in 15.5 percent of HIV-positive patients with CD4+ counts <100 cells/µl (i.e., 16 out of 103 patients). Fundus photography mediated telescreening is less successful, with a lower pick-up rate of 5.8 percent (i.e., six out of 103 patients). Use of ultra-widefield retinal imaging may improve the success rate of telescreening.24
In conclusion, CMVR is a sight-threatening infection caused by a member of the HHV family.5,6,9 Both HIV and CMVR are more common in the developing world.9-11,38 The gold-standard in CMVR therapy is systemic antiviral medication (e.g., valganciclovir).17 But due to cost, CMVR is still treated by intravitreal antivirals alone (e.g., ganciclovir) in many settings.13,31,32,36 REVIEW
Dr. Yeung is a uveitis and ocular immunology fellow at the National Eye Institute, Bethesda, Md. Dr. Downes is a cornea fellow at The Casey Eye Institute, Oregon Health Science University, Portland, Ore. Dr. Sen is the director of the uveitis and ocular immunology fellowship program at the NEI and has a joint appointment as a clinical professor in the Department of Ophthalmology at George Washington University, Washington, D.C. Dr. Cunningham is the director of the Uveitis Service at California Pacific Medical Center, San Francisco; an adjunct clinical professor of ophthalmology at Stanford University School of Medicine; a research associate at the Francis I. Proctor Foundation, UCSF School of Medicine, San Francisco; and a partner in the West Coast Retina Medical Group, San Francisco.
Correspondence should be directed to Dr. Sen at senh@nei.nih.gov. This work was supported by the NEI Intramural Research Program.
1. Cunningham ET, Jr., Hubbard LD, Danis RP, et al. Proportionate topographic areas of retinal zones 1, 2, and 3 for use in describing infectious retinitis. Arch Ophthalmol 2011;129:1507-1508.
2. Whitcup SM. Acquired Immunodeficiency Syndrome. In R. B. Nussenblatt and S. M. Whitcup. Uveitis: Fundamentals and Clinical Practice, Fourth Edition. London: Mosby, 2010:161-175.
3. Ausayakhun S, Keenan JD, Ausayakhun S, et al. Clinical features of newly diagnosed cytomegalovirus retinitis in northern Thailand. Am J Ophthalmol 2012;153:923-931 e921.
4. Cunningham ET, Jr., Wong RW, Takakura A, et al. Necrotizing herpetic retinitis. Ocul Immunol Inflamm 2014;22:167-169.
5. Kozak I, McCutchan A, Freeman WR. HIV-Associated Infections. In S. J. Ryan. Retina, Fifth Edition. London: Elsevier Saunders, 2013:1441-1472.
6. Zamora MR. DNA viruses (CMV, EBV, and the herpes viruses). Sem Resp Crit Care Med 2011;32:454-470.
7. Holland GN, Vaudaux JD, Jeng SM, et al. Characteristics of untreated AIDS-related cytomegalovirus retinitis. I. Findings before the era of highly active antiretroviral therapy (1988 to 1994). Am J Ophthalmol 2008;145:5-11.
8. Yeh S, Forooghian F, Faia LJ, et al. Fundus autofluorescence changes in cytomegalovirus retinitis. Retina 2010;30:42-50.
9. Cunningham ET, Jr. Cytomegalovirus: Ophthalmic perspectives on a pervasive pathogen. Expert Rev Ophthalmol 2011;6:489-491.
10. Korndewal MJ, Mollema L, Tcherniaeva I, et al. Cytomegalovirus infection in the Netherlands: Sero-prevalence, risk factors, and implications. J Clin Virol 2015;63:53-58.
11. Seale H, MacIntyre CR, Gidding HF, et al. National serosurvey of cytomegalovirus in Australia. Clin Vaccine Immunol 2006;13:1181-1184.
12. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010;20:202-213.
13. Chan TS, Cheung CY, Yeung IY, et al. Cytomegalovirus retinitis complicating combination therapy with rituximab and fludarabine. Ann Hematol 2015;94:1043-1047.
14. Jap A, Chee SP. Cytomegalovirus-associated anterior segment infection. Expert Review of Ophthalmology 2011;6:517-528.
15. Jeon S, Lee WK. Cytomegalovirus Retinitis in a Human Immunodeficiency Virus-negative Cohort: Long-term Management and Complications. Ocul Immunol Inflamm 2015:1-8.
16. Friedman AH, Freeman WR, Orellana J, et al. Cytomegalovirus retinitis and immunodeficiency in homosexual males. Lancet 1982;1:958.
17. Jabs DA, Ahuja A, Van Natta ML, et al. Long-term Outcomes of Cytomegalovirus Retinitis in the Era of Modern Antiretroviral Therapy: Results from a United States Cohort. Ophthalmology 2015;122:1452-63.
18. Urban B, Bakunowicz-Lazarczyk A, Michalczuk M. Immune recovery uveitis: Pathogenesis, clinical symptoms, and treatment. Mediators Inflamm 2014;2014:971417.
19. Downes K, Tarasewicz DG, Weisberg LJ, et al. Cytomegalovirus Retinitis in HIV-negative Patients. J Ophthalmic Inflamm Infect (submitted). 2015.
20. Sen HN, Robinson MR, Fischer SH. CMV retinitis in a patient with Good syndrome. Ocul Immunol Inflamm 2005;13:475-478.
21. Vannozzi L, Bacherini D, Sodi A, et al. Cytomegalovirus retinitis following intravitreal dexamethasone implant in a patient with central retinal vein occlusion. Acta Ophthalmol 2016 Mar;94(2):e158-60.
22. Takakura A, Tessler HH, Goldstein DA, et al. Viral retinitis following intraocular or periocular corticosteroid administration: A case series and comprehensive review of the literature. Ocul Immunol Inflamm 2014;22:175-182.
23. Yen M, Chen J, Ausayakhun S, et al. Retinal detachment associated with AIDS-related cytomegalovirus retinitis: Risk factors in a resource-limited setting. Am J Ophthalmol 2015;159:185-192.
24. Jirawison C, Yen M, Leenasirimakul P, et al. Telemedicine screening for cytomegalovirus retinitis at the point of care for human immunodeficiency virus infection. JAMA Ophthalmol 2015;133:198-205.
25. Yeh S, Fahle G, Forooghian F, et al. Polymerase chain reaction-based ganciclovir resistance testing of ocular fluids for cytomegalovirus retinitis. Arch Ophthalmol 2012;130:113-115.
26. Schneider EW, Elner SG, van Kuijk FJ, et al. Chronic retinal necrosis: Cytomegalovirus necrotizing retinitis associated with panretinal vasculopathy in non-HIV patients. Retina 2013;33:1791-1799.
27. Mathur G, Ratra D, Bhuibhar SS, et al. Clinical Outcomes of Retinal Detachment Surgery following Cytomegalovirus Retinitis in Patients on Highly Active Anti-retroviral Therapy for Acquired Immune Deficiency Syndrome. Ocul Immunol Inflamm 2015;23(5):400-4.
28. Garcia RF, Flores-Aguilar M, Quiceno JI, et al. Results of rhegmatogenous retinal detachment repair in cytomegalovirus retinitis with and without scleral buckling. Ophthalmology 1995;102:236-245.
29. Bakshi NK, Fahle GA, Sereti I, et al. Cytomegalovirus retinitis successfully treated with ganciclovir implant in a patient with blood ganciclovir resistance and ocular ganciclovir sensitivity. Eye (Lond) 2012;26:759-760.
30. Vishnevskia-Dai V, Shapira Y, Rahav G, et al. Cytomegalovirus retinitis in HIV-negative patients: A practical management approach. Ophthalmology 2015;122:866-868 e863.
31. Arevalo JF, Garcia RA, Mendoza AJ. High-dose (5,000-microg) intravitreal ganciclovir combined with highly active antiretroviral therapy for cytomegalovirus retinitis in HIV-infected patients in Venezuela. Eur J Ophthalmol 2005;15:610-618.
32. Smith CL. Local therapy for cytomegalovirus retinitis. Ann Pharmacother 1998;32:248-255.
33. Hoang QV, Simon DM, Kumar GN, et al. Recurrent CMV retinitis in a non-HIV patient with drug-resistant CMV. Graefe’s Arch Clin Exp Ophthalmol 2010;248:737-740.
34. Morlet N, Young S, Naidoo D, et al. High dose intravitreal ganciclovir injection provides a prolonged therapeutic intraocular concen



