 |
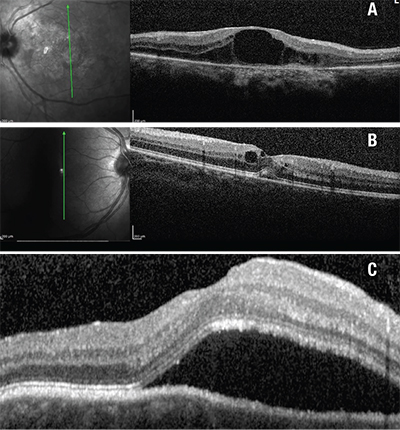
| Figure 1. Different forms of macular edema in uveitis. 1A (top). Cystoid macular edema in serpiginous choroidopathy. 1B (middle). Diffuse macular thickening with cystic changes in chronic uveitis. 1C (bottom). Subretinal fluid in Vogt-Koyanagi-Harada disease. |
Given its high degree of reproducibility, SD-OCT is an invaluable technique for characterizing pathologic features of the retina, including the retinal pigment epithelium, and in assessing disease activity and therapeutic response. Enhanced-depth- imaging OCT now allows better visualization of the choroid. This review summarizes the applications for SD-OCT imaging in various ocular inflammatory diseases.
Edema, ERM and CNV
Detection and monitoring of uveitic macular edema using SD-OCT has been extensively studied. The 12-line radial and 25-line raster scans are comparable in their ability to detect intraretinal and subretinal fluid. Three different patterns of fluid distribution in the macular have been described: cystoid macular edema (See Figure 1A); diffuse macular edema (See Figure 1B); and serous retinal detachment (See Figure 1C). CME appears as low reflective intraretinal spaces that are separated by thin retinal tissue with high reflectivity. DME shows small areas of hyporeflectivity and a spongy appearance to the retinal layers, thus resulting in increased macular thickness. Lastly, serous retinal detachments are characterized by a separation between the neurosensory retina and RPE.1 Isolated anterior uveitis usually causes non-cystic retinal thickening that correlates well with disease activity.2
OCT can be used to quantitate response to therapy. One study that showed a 20-percent change in retinal thickness correlated well with a 10-letter change in visual acuity, suggesting that changes in SD-OCT could be a meaningful measure of treatment success in macular edema related to uveitis.3
Epiretinal membrane formation is common in uveitis and appears on SD-OCT as jagged hyperreflective lines adherent to the inner-most layers of the retina. ERM formation is often found in conjunction with vitreoretinal traction, and a tractional mechanism may contribute to the onset of macular edema in uveitis.1 The vitreomacular interface may also affect response to therapy for uveitic macular edema, with eyes having posterior vitreous detachments showing greater and faster response to intravitreal corticosteroids or bevacizumab than eyes with vitreomacular adhesion.4
Choroidal neovascularization is a leading cause of impaired vision and blindness in eyes with uveitis, and SD-OCT is a useful way to detect it and monitor treatment (See Figure 2).
White Dot Syndromes
SD-OCT can identify hyperreflectivity, thinning, loss of or edema of retinal and chorioretinal interface layers in white dot syndromes, which may provide more accurate case definitions of these disorders and better prognostic clues. For example, restoration of retinal architecture at the IS/OS junction following systemic immunomodulatory treatment has been reported in birdshot chorioretinopathy.5 In contrast, there is no anatomic or visual field improvement in regions with initial photoreceptor loss in acute zonal occult outer retinopathy (AZOOR).1
However, OCT findings may not predict visual outcomes. In acute posterior multifocal placoid pigment epitheliopathy, for example, photoreceptor atrophy and continued disruption of the IS/OS junction or RPE can still be seen in later phases despite visual recovery.6
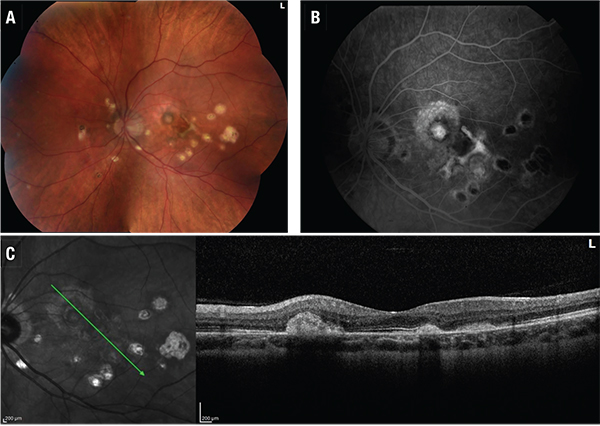 |
| Figure 2. Punctate inner choroidopathy with choroidal neovascularization, left eye. 2A (top, left). Color fundus photo. 2B (top, right). Fluorescein angiogram. 2C (above). Spectral-domain showing choroidal neovascular membrane. |
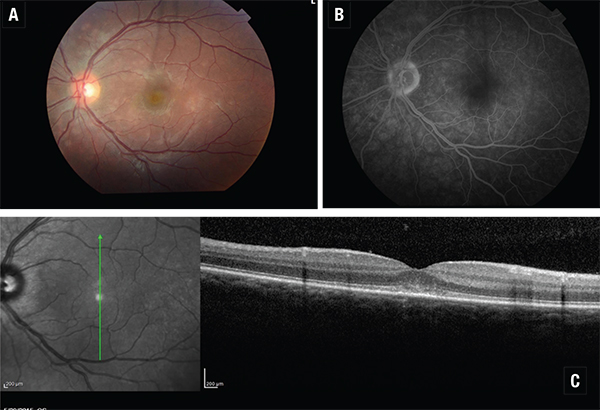 |
| Figure 3. Multiple evanescent white dot syndrome. 3A (top, left). Color fundus photo, left eye. 3B (top, right). Fluorescein angiogram, left eye. 3C (bottom). Spectral-domain optical coherence tomography, left eye. Note the disruption of the subfoveal ellipsoid region. |
SD-OCT of macular lesions in punctate inner choroidopathy shows elevation of the RPE with sparing of Bruch’s membrane and choroid. Bands corresponding to photoreceptors also appear compressed during active phases and return to normal caliber with clinical stabilization.8 Both active and inactive serpiginous lesions demonstrate increased reflectivity in the choroid and deep retinal layers in addition to disruption at the IS/OS junction. In MEWDS, multifocal debris centered at and around the ellipsoid layer, protruding toward the outer nuclear layer corresponds to the location of dots seen with photography, indocyanine green angiography, and fluorescein angiography. (See Figure 3)9
Posterior Uveitis
Although occlusive retinal vasculitis is a cardinal finding in Behçet’s disease, choroidal thickening may be related to vasculitic processes affecting choroidal vasculature. Studies using SD-OCT to focus on foveal changes during remission of Behçet uveitis have revealed better visual acuity and greater foveal thickness in eyes with an intact IS/OS line compared with those with poorly defined architecture.10
SD-OCT can easily identify structural changes of the retina and choroid in VKH disease. Intraretinal fluid accumulation in serous retinal detachments causes outer segment detachment from the photoreceptor layer. The subsequent fibrin precipitation in the fluid space of the outer photoreceptor layer forms membranes and the cystoid spaces that are typically seen in active disease.11
Enhanced-depth imaging has made it possible to visualize the choroidal involvement in VKH disease. A reduction in the number of hyperreflective dots at the inner choroid compared to normal controls has been attributed to non-perfusion of small choroidal vessels due to inflammatory cell infiltration and stromal scarring-induced dropout of similar vessels in the active and convalescent stages of VKH disease, respectively.12 An increase in choroidal thickness during the acute phase has been well-documented, as has the return to normal values upon effective treatment with corticosteroid therapy.
Infectious Posterior Uveitis
Acute syphilitic posterior placoid chorioretinopathy assessed by SD-OCT in one study demonstrated transient sub-foveal fluid one to two days after presentation in nearly half the eyes that were examined. After seven days, irregular hyperreflectivity and nodular elevations between the photoreceptors and RPE as well as segmental loss of the IS/OS band were consistently noted (See Figures 4A & 4B). In some cases, loss of the external limiting membrane and punctate, hyperreflective spots in the choroid were observed. With the initation of neurosyphilis therapy, all of these findings were resolved by one-month follow-up.13
Ocular toxoplasmosis is the most frequent cause of infectious posterior segment inflammation. In one series of patients with active toxoplasmic retinochoroiditis, SD-OCT exhibited increased retinal thickness with and without sub-retinal fluid accumulation. Inner retinal layer reflectivity also resulted in RPE and choriocapillaris shadowing. Other pertinent findings include persistent disorganization of retinal layers secondary to scar formation and a trend toward separation of the hyaloid at six-week follow-up.14
Anterior Uveitis
Slit-lamp examination remains the standard method for evaluating anterior chamber inflammation, but suffers from limited interobserver agreement, lack of a non-linear grading scale and variability in illumination from one slit lamp to another. While SD-OCT currently has limited applications in anterior uveitis, SD-OCT has axial and transverse resolution finer than the diameter of a white blood cell (7 to 9 μm), and can be modified to create three-dimensional images of the anterior chamber. Using these techniques, grading of cells by anterior segment OCT has been shown to correlate strongly with clinical grading.15 A potential limitation of this technique is the misidentification of pigment in the anterior chamber for white blood cells, although this can be a problem in slit-lamp grading as well.
Limitations
There are limitations of OCT include potential lack of reproducibility when normative values may not be known (e.g., children with uveitis) or there is more inherent variability of the tissue being assessed (e.g., optic nerve head and choroid vs. macula). Uveitis may affect the interpretation of OCT in other disorders such as glaucoma. Uveitis may cause falsely elevated estimates of retinal nerve fiber layer thickness; continued thinning of the RNFL and increased cupping, despite good intraocular pressure control in such eyes, may occur from resolution of edema of the RNFL as the uveitis resolves.16 Therefore, screening for glaucomatous RNFL changes should be performed only when the uveitis is inactive. Finally, despite its many advantages, OCT has not replaced fluorescein angiography for evaluation of macular edema. OCT (preferable for assessing macular thickness) and FA (preferable for assessing macular leakage) show only moderate agreement regarding macular edema status in uveitis. Macular leakage cannot be ruled out if macular thickness is absent, so that FA may be necessary if the OCT is normal when detection of leakage would alter management.17
Recent Advances
Three-dimensional OCT can produce volumetric renderings with excellent resolution of the retina and retinal pigment epithelium. While such images have been mostly used in the evaluation of vitreoretinal interface disorders, central serous retinopathy and ocular tumors, they may eventually be useful in diagnosis and management of infectious chorioretinopathy (e.g., fungal, tuberculous).
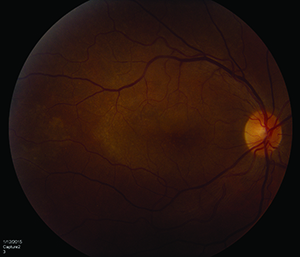 | Figure 4. Placoid syphilitic retinitis, right eye. 4A. Color fundus photo. 4B. Spectral-domain optical coherence tomography showing outer retinal changes. |
 | |
“En face” OCT is a new technique with particular applicability for imaging of disorders involving the IS/OS photoreceptor junction and the inner choroid. In one case report of a patient with MEWDS, en face OCT showed attenuation of these layers in a pattern that mirrored hypofluorescent spots seen with ICG angiography.18 En face OCT highlighted confluent areas of middle retina hyper-reflectivity corresponding to these lesions. Three distinct en face OCT patterns were observed: arteriolar; fern-like; and globular. Microperimetry demonstrated relative scotomas mapping to the area of middle retinal hyperreflectivity seen on en face OCT.
OCT of the optic nerve and peripapillary retina is frequently used in the assessment of disorders such as papilledema, optic neuritis and glaucoma. One group described the use of OCT to measure peripapillary RNFL thickness and optic nerve central thickness in monitoring resolution of inflammatory papillitis.19
OCT angiography is a non-invasive means of assessing retinal and choroidal blood flow and can detect and quantify neovascularization and capillary dropout without the use of dye. Four layers are assessed en face: the superficial retinal vascular plexus (similar to the traditional fluorescein angiogram view); the deep retinal vascular plexus; the outer retina; and the choriocapillaris. Currently, OCTA is limited by its ability to image only the posterior pole. However, the combination of high-definition SD-OCT and OCTA has allowed a detailed understanding of variable areas of capillary dropout within the superficial and deep retinal capillary plexi in the inner retina in patients with vascular disorders causing paracentral acute middle maculopathy.20 Additional experience will show to what extent such modifications of OCT can replace invasive imaging in the diagnosis, natural history and response to treatment in uveitic white dot syndromes and other inflammatory chorioretinal interface diseases such as birdshot retinopathy, with or without choroidal neovascularization.21
Advances in OCT for in vivo imaging of the retina have allowed for improved quantitative and qualitative assessment of uveitis-related pathology. High-resolution OCT imaging has led to better understanding of distinct changes at the vitreoretinal interface, retinal layers and choroid that are specific to various inflammatory entities. The integrity of the IS/OS line has also been shown to be a valuable predictor of visual recovery, treatment response and prognosis. Consequently, SD-OCT has become a standard ancillary test for detecting and monitoring uveitic ocular disease. It remains unclear to what degree the knowledge SD-OCT has provided about these disorders will improve prognostic skills, eliminate the role for invasive procedures such as fluorescein or indocyanine angiography, or allow clinicians to distinguish more confidently those syndromes for which no treatment is necessary (e.g., MEWDS) from those in which long-term immunosuppression is typically indicated (e.g., MFC-PU, birdshot chorioretinopathy). REVIEW
The authors practice in the Uveitis Unit/Retina Division at Wills Eye Hospital, where Dr. Dunn is the director of the division. He is a professor of ophthalmology at the Sidney Kimmel Medical College, Thomas Jefferson University. Contact him at 840 Walnut St., Suite 1020, Philadelphia, PA 19107. Phone: (215) 928-3095; fax: (215) 928-3484. Email: jpdunn@willseye.org.
The authors have no financial disclosures to report.
1. Onal S, Tugal-Tutkun I, Neri P, et al. Optical coherence tomography imaging in uveitis. Int Ophthalmol 2014;34(2):401-35.
2. Castellano CG, Stinnett SS, Mettu PS, et al. Retinal thickening in iridocyclitis. Am J Ophthalmol 2009;148:341-9.
3. Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group et al. Identifying a clinically meaningful threshold for change in uveitic macular edema evaluated by optical coherence tomography. Am J Ophthalmol 2011;152:1044-1052.
4. Munk MR, Ram R, Rademaker A, et al. Influence of the vitreomacular interface on the efficacy of intravitreal therapy for uveitis-associated cystoid macular oedema. Acta Ophthalmol 2015 Feb 23. doi: 10.1111/aos.12699. [Epub ahead of print].
5. Forooghian F, Gulati N, Jabs DA. Restoration of retinal architecture following systemic immunosuppression in birdshot chorioretinopathy. Ocul Immunol Inflamm 2010;18(6):470-1.
6. Cheung CM, Yeo IY, Koh A. Photoreceptor changes in acute and resolved acute posterior multifocal placoid pigment epitheliopathy documented by spectral-domain optical coherence tomography. Arch Ophthalmol 2010;128:644-6.
7. Spaide RF, Koizumi H, Freund KB. Photoreceptor outer segment abnormalities as a cause of blind spot enlargement in acute zonal occult outer retinopathy-complex diseases. Am J Ophthalmol 2008;146:111-20.
8. Channa R, Ibrahim M, Sepah Y, et al. Characterization of macular lesions in punctate inner choroidopathy with spectral domain optical coherence tomography. J Ophthalmic Inflamm Infect 2012;2(3):113-20.
9. Marsiglia M, Gallego-Pinazo R, Cunha de Souza E, et al. Expanded clinical spectrum of multiple evanescent white dot syndrome with multimodal imaging. Retina 2015 jul 8. [epub ahead of print].
10. Unoki N, Nishijima K, Kita M, et al. Structural changes of fovea during remission of Behçet’s disease as imaged by spectral domain optical coherence tomography. Eye (Lond) 2010;24(6):969-75.
11. Ishihara K, Hangai M, Kita M, et al. Acute Vogt-Koyanagi-Harada disease in enhanced spectral-domain optical coherence tomography. Ophthalmology 2009;116:1799-807.
12. Fong AH, Li KK, Wong D. Choroidal evaluation using enhanced depth imaging spectral-domain optical coherence tomography in Vogt-Koyanagi-Harada disease. Retina 2011;31:502-9.
13. Pichi F, Ciardella AP, Cunningham ET Jr, et al. Spectral domain optical coherence tomography findings in patients with acute syphilitic posterior placoid chorioretinopathy. Retina 2014;34:373-84.
14. Diniz B, Regatieri C, Andrade R, et al. Evaluation of spectral domain and time domain optical coherence tomography findings in toxoplasmic retinochoroiditis. Clin Ophthalmol 2011;5:645-50.
15. Sharma S, Lowder CY, Vasanji A, et al. Automated analysis of anterior chamber inflammation by spectral-domain optical coherence tomography. Ophthalmology 2015;122:1464-70.
16. Asrani S, Moore DB, Jaffe GJ. Paradoxical changes of retinal nerve fiber layer thickness in uveitic glaucoma. JAMA Ophthalmol 2014;132:877-880.
17. Kempen JH, Sugar EA, Jaffe GJ, et al. Fluorescein angiography versus optical coherence tomography for diagnosis of uveitic macular edema. Ophthalmology 2013;120:1852-9.
18. Combillet F, Rougier MB, Blaizeau M, et al. “En face” optical coherence tomography for multiple evanescent white dot syndrome. [French] J Fr Ophtalmol 2013;36:652-7.
19. Cho H, Pillai P, Nicholson L, Sobrin L. Inflammatory papillitis in uveitis: Response to treatment and use of optic nerve optical coherence tomography for monitoring. Ocul Immunol Inflamm 2015;24:1-13. [Epub ahead of print].
20. Rahimy E, Kuehlewein L, Sadda SR, Sarraf D. Paracentral acute middle maculopathy: What we knew then and what we know now. Retina 2015 [ePub ahead of print].
21. de Carlo TE, Bonini Filho MA, Adhi M, Duker JS. Retinal and choroidal vasculature in birdshot chorioretinopathy analyzed using spectral domain optical coherence tomography angiography. Retina 2015 Sep 7. [Epub ahead of print].