|
Embryonic Stem Cells
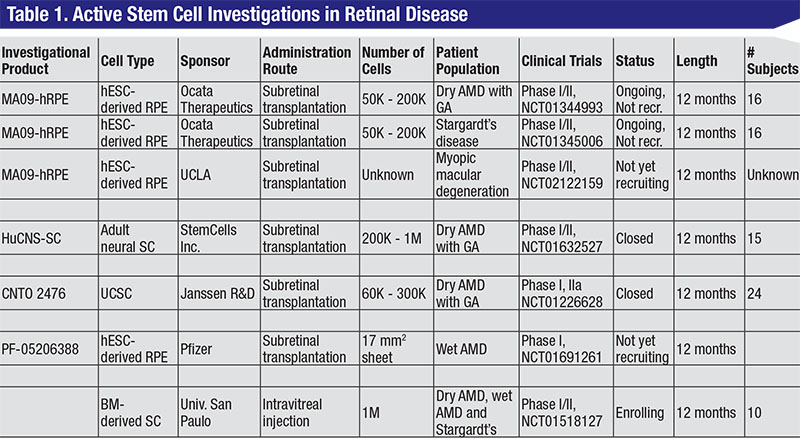
Human embryonic stem cells (hESC) are the earliest and most basic component of our bodies. They are pluripotent and have the ability to differentiate into all cell types of the body. In theory, hESCs can be made into specific retinal cell types and used to replenish degenerating or malfunctioning cell in many disease states. Animal studies have shown retinal pigment epithelium derived from hESCs can preserve vision after transplantation.2 Ocata Therapeutics Inc. (formerly Advanced Cell Technology) has been running a clinical program since 2011 with a Phase I/II open-label, multicenter, nonrandomized, prospective study to determine the safety and potential efficacy of subretinal RPE cells spontaneously produced from hESCs (MA09-hRPE). In these trials, patients with GA in dry AMD (NCT01344993) and Stargardt’s disease (NCT01345006) had pars plana vitrectomies and were subretinally transplanted with 50,000 to 200,000 hESC-derived RPE cells.
In a recent report, nine eyes in patients with GA and nine eyes in patients with Stargardt’s disease were transplanted and followed for a median of 22 months.3 No treated eyes developed abnormal tissue proliferation, teratoma formation, rejection or inflammation. Of treated eyes, 72 percent had patches of increasing subretinal pigmentation consistent with viable, transplanted RPE. Visual acuity increased in 10 of 18 eyes and decreased in one eye. The remaining seven eyes showed no change in vision at 22 months.
Induced Pluripotent Stem Cells
Induced Pluripotent Stem (iPS) cells are adult cells, such as fibroblasts, that have been genetically reprogrammed to behave like hESCs. Thus, they are fully mature tissue cells that are “regressed” in vitro to become pseudo-hESCs. iPS cells are able to replicate themselves and are pluripotent enough to differentiate into any cell type, just like hESCs. However, it is not known whether they differ in clinically meaningful ways from hESCs.
One potential benefit of iPS cells is that they can be induced from cells harvested from an individual patient. Once prepared, those same cells can be transplanted back into the same person—theoretically reducing the risk of immune rejection.
The Laboratory for Retinal Regeneration at the RIKEN Center in Kobe, Japan has initiated a trial in AMD using iPS-derived RPE cells in sheets. This is the first trial to use iPS cells in AMD and is ongoing. Final results have yet to be reported.4-6
 |
Adult Stem Cells
Like embryonic stem cells, adult stem cells are undifferentiated and can self-renew. However, adult stem cells are not pluripotent and can only differentiate into specific tissue lineages. Theoretically, this reduces the risk of teratoma formation and other hESC-related side effects.
One active eye program that is using adult stem cells is managed by StemCells Inc., which is conducting a Phase I/II study to assess the safety and preliminary efficacy of adult human central nervous system stem cells (HuCNS-SC) in AMD with GA (NCT01632527). HuCNS-SCs were found in pre-clinical models to preserve photoreceptors in the RCS rat model and as adult stem cells, HuCNS-SCs can theoretically only differentiate into cells in the neural lineage.7
In the study, pars plana vitrectomy was performed and HuCNS-SCs were subretinally transplanted. Subjects received either 200,000 or 1 million HuCNS-SCs and were enrolled in two cohorts—one with 20/400 or worse vision and the second with 20/320 to 20/100 vision. The study is now closed to enrollment with 15 subjects from three U.S. clinical sites.
Preliminary data presented to date suggested that the these transplanted cells have not been associated with any adverse reactions. There has been no tumor formation or epiretinal membrane proliferation reported. Eyes that received HuCNS-SCs also had better contrast sensitivity and visual acuity when compared to the fellow control eye. Lastly, treated eyes had about a 70-percent reduction in GA growth rate when compared to the fellow control eye. There is a controlled Phase II study planned to begin this year.
Umbilical Cord Stem Cells
Stem cells from the umbilical cord can contribute to blood and mesenchymal tissue lineages and have the potential to slow degeneration by releasing growth or trophic factors. One such program is being run by Janssen Biotech Inc. The investigational product, CNTO 2476, has been shown to slow vision loss in the RCS rat model after subretinal transplantation.8 A Phase I trial was performed to treat patients with RP (NCT00458575), but this was halted in 2010. The same year, a Phase I/II trial was initiated to assess the safety and preliminary efficacy of these cells in the subretinal space of patients with GA in dry AMD (NCT01226628). In the study, 60,000 to 300,000 cells were subretinally administered in 24 patients using an iTrack Model 275 microcathether.
|
Bone Marrow Stem Cells
Bone Marrow Stem Cells (BMSC) have been shown to rescue retinal degeneration in mouse models.9,10 Early clinical trials were conducted to evaluate the short-term safety (10 months) of 1 million cells in three patients with retinitis pigmentosa and two patients with cone-rod dystrophy.11,12 No detectable structural or functional toxicity was observed. Current studies included intravitreal injections of 1 million BMSCs in RP patients in Brazil (NCT01560715) and Thailand (NCT01531348), AMD patients in Brazil (NCT01518127) and ischemic retinopathy patients in Brazil (NCT01518842). Results have yet to be reported.
The use of stem cells to treat retinal disease has gone from the theoretical to proof of concept to controlled clinical trials. The next few years will be telling and should provide adequate data to determine whether this treatment approach will be both efficacious and well-tolerated. REVIEW
Dr. Leng is the director of ophthalmic diagnostics at the Byers Eye Institute at Stanford and is a clinical assistant professor of ophthalmology at the Stanford University School of Medicine. He is an investigator for StemCells Inc. He can be reached via phone: (650) 498 4264; fax: (888) 565 2640; or e-mail: tedleng@stanford.edu.
1. Stem Cell Basics: Introduction. In Stem Cell Information [World Wide Web site]. Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services, 2002 [cited Wednesday, February 18, 2015] Available at <http://stemcells.nih.gov/info/basics/pages/basics1.aspx>
2. Rowland TJ, Buchholz DE, Clegg DO. Pluripotent human stem cells for the treatment of retinal disease. J Cell Physiol 2012;227(2):457-466.
3. Schwartz SD, Regillo CD, Lam BL et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2014;385(9967):509-516.
4. Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol 2008;26(2):215-224.
5. Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett 2009;458(3):126-131.
6. Jin Z, Okamoto S, Osakada F, et al. Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PLoS ONE 2011;6(2):e17084.
7. Mcgill TJ, Cottam B, Lu B, et al. Transplantation of human central nervous system stem cells – neuroprotection in retinal degeneration. Eur J Neurosci 2012;35(3):468-477.
8. Lund RD, Wang S, Lu B, et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells 2007;25(3):602-611.
9. Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefe’s Arch Clin Exp Ophthalmol 2007;245(3):414-422.
10. Otani A, Dorrell Mi, Kinder K, et al. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest 2004;114(6):765-774.
11. Siqueira RC, Messias A, Voltarelli JC, Scott IU, Jorge R. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: A Phase I trial. Retina 2011;31:1207-1214.
12. Moore AT. Cone and cone-rod dystrophies. J Med Genet 1992;29(5):289-290.