A variety of pharmacologic agents can cause toxicity to the retina. While many of these can be grouped by anatomic location or type of toxicity, there are also individual medications with characteristic effects. In this article, we will describe several major categories of pharmacologic retinal toxicity and discuss examples of individual medications.
Pigmentary Retinopathy
• Quinolines. Chloroquine (Aralen) and hydroxychloroquine (Plaquenil) are traditional antimalarial agents now used in the treatment of autoimmune diseases, including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Both medications have been shown to bind melanin and to concentrate in the iris, ciliary body and retinal pigment epithelium, altering normal physiologic function.1
Early on, patients may be entirely asymptomatic, with only blunting of the foveal reflex and RPE granular pigmentary changes. With progression, symptoms can include blurred vision, decreased vision, scotomas and photopsias.
As RPE degeneration continues, the classic fundus pattern of bilateral bulls-eye maculopathy can be identified on both clinical and fluorescein angiography exam. In late-stage disease, optic disc pallor and arteriovenous narrowing may also develop.
Retinal toxicity has been shown in patients taking greater than 3 mg/kg/day of chloroquine or 6.5 mg/kg/day of hydroxychloroquine. While most cases of toxicity have been reported for chloroquine, hydroxychloroquine has supplanted it as the more commonly used treatment agent for both RA and SLE.2
The American Academy of Ophthalmology has suggested guidelines in order to monitor patients on hydroxychloroquine therapy, recommending a baseline dilated fundus exam, automated macular perimetry and Amsler grid testing.3 Patients should be monitored annually, as toxicity can be irreversible and even progress after drug cessation.
• Thioridazine. Thioridazine (Mellaril) is a piperadine antipsychotic agent that has seen a decrease in use with the advent of atypical antipsychotic agents. Ocular toxicity was first described in 1980 and has been shown in both short- and long-term treatment.4
Symptoms of toxicity include decreased vision and dyschromatopsia. On clinical exam, macular pigmentary changes can develop into a "salt-and-pepper" pattern. Long-term use can cause significant optic nerve atrophy and destruction of both the RPE and choriocapillaris.
Once signs or symptoms are noted, the medication should be discontinued. Unfortunately, complete visual recovery is rare, and visual dysfunction often persists and may even progress after cessation.
• Deferoxamine. Deferoxamine (Desferal) is an iron-chelating agent used to treat conditions with excessive serum iron levels, including hemochromatosis. Given in both subcutaneous and intravenous forms, it can also be used to treat aluminum toxicity.
Ocular signs of toxicity include vision loss, scotomas, dyschromatopsia, and nyctalopia.
Fundus examination is often normal; however, RPE mottling may develop over time. Studies have suggested that deferoxamine toxicity may cause diminished ERG amplitudes and late-phase vascular leakage on FA.5
Deferoxamine toxicity is reversible with drug cessation, with full recovery of visual function. However, the medication is often used for chronic conditions, such as thalassemia, that require regular therapy. In these patients, monitoring with frequent ophthalmic exams is essential to detect early vision loss.
Choroidal Toxicity
• Topiramate. Topiramate (Topamax) is a sulfamate-substituted monosaccharide oral anticonvulsant used for the treatment of seizures, prophylaxis for migraine, as well as off-label in the treatment of bipolar disorder. Since 2001, several case reports have described induced myopia and bilateral angle-closure glaucoma associated with topiramate use.6
Patients often present within one month of treatment onset, complaining of blurred vision, eye pain and headache. Signs include diffuse corneal edema, shallowing of the anterior chamber and significantly raised intraocular pressure. Retinal striae on fundus exam may also be noted.
It has been suggested that uveal effusion or ciliary edema leads to forward displacement of the lens-iris diaphragm and thickening of the lens by relaxation of zonules. This, in turn, causes anterior chamber shallowing and induced myopia, while retinal striae are caused by vitreoretinal traction.7
While all of the changes are reversible with prompt cessation of the drug, it is often necessary to use cycloplegic agents to reverse the anterior displacement of the lens-iris diaphragm. Laser peripheral iridotomy is not useful in lowering IOP as the mechanism of angle-closure is not pupillary block. Instead, a combination of topical anti-glaucoma agents can be used for IOP control.
• Metronidazole. Other sulfa-containing medications can cause a similar pattern of symptoms. Metronidazole (Flagyl) is a nitroimadazole sulfa agent used to treat anaerobic and protozoal infections, such as Clostridium and Trichomonas, respectively.8It, along with sulfa analogs such as sulfanilamide (AVC), acetazolamide (Diamox) and hydrochlorothiazide (Oretic), has been shown to cause induced bilateral myopia and anterior chamber shallowing from ciliochoroidal swelling.9 As with topiramate, stopping each medication will lead to resolution of ocular changes.
Macular Edema
|
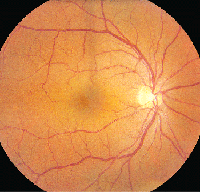
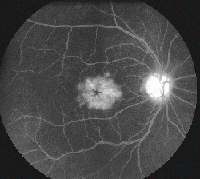
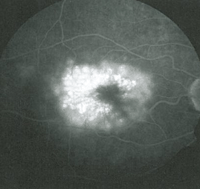
• Latanoprost. Latanoprost (Xalatan) is a prostaglandin analog used to lower IOP by increasing uveoscleral outflow. Patients may present with ocular side effects including conjunctival hyperemia, darkening of eyelashes and iris heterochromia. In addition, latanoprost is a factor in the development of reversible cystoid macular edema (See Figures 1a & b).
The risk has been shown to be particularly high in patients with a prior history of complicated intraocular surgery, epiretinal membrane, anterior uveitis, or diabetes mellitus.10 The CME is reversible on discontinuation of the latanoprost but resolution can be expedited with the use of topical steroidal and non-steroidal agents.
• Epinephrine. The sympathomimetic agent epinephrine, and its prodrug dipivefrin, is no longer used as primary therapy for the treatment of glaucoma in the United States. It acts to lower IOP by decreasing aqueous production but has been shown to induce cystoid macular edema in glaucomatous aphakic or pseudophakic patients.11
Patients complain of blurred or decreased vision within weeks to months of initiating therapy. CME can be documented by FA and ocular coherence tomography and will resolve with drug cessation. Consequently, epinephrine and dipivefrin should be avoided as first-line therapy for treatment of elevated IOP in patients with previous intraocular surgery.
• Niacin. Niacin, or vitamin B3, is used to treat pellagra, hyperlipidemia and hypercholesterolemia. While facial flushing is the most common systemic side effect, patients may have visual complaints including blurred vision, decreased vision and metamorphopsia.
On clinical exam, the macula appears edematous. However, niacin-induced macular edema has the unique characteristic of being angiographically silent. While OCT imaging exhibits classic cystic intraretinal edema, FA is silent without leakage, which is thought to indicate that the edema is secondary to fluid accumulation inside retinal cells, as opposed to an extracellular location.12
The discontinuation of niacin will gradually reduce CME and improve visual acuity.
• Rosiglitazone. Rosiglitazone (Avandia) is a thiazolidinedione oral agent used to decrease insulin resistance in type 2 diabetes. In 2005, a case report described worsening diabetic macular edema with rosiglitazone use.13
While congestive heart failure and renal disease are known to worsen DME, systemic fluid retention and peripheral edema have also been described in up to 15 percent of patients on therapy.14,15 Rosiglitazone may independently increase retinal endothelial cell permeability and increase vascular endothelial growth factor, leading to intraretinal edema and blurred vision.16
Drug cessation may lead to rapid resolution of both the DME and peripheral edema, however, most cases resolve slowly and may require focal laser photocoagulation as adjunctive therapy.
Crystalline Retinopathy
Crystalline retinopathy has been observed in several systemic and inherited renal diseases including cystinosis and oxalosis. The appearance of refractile crystals in the subretinal, intraretinal or preretinal spaces has also been associated with medication use.
|
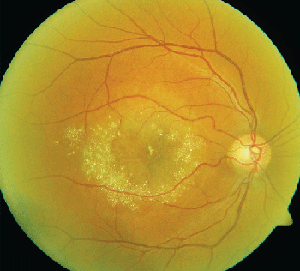
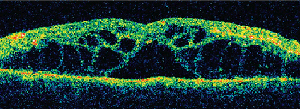
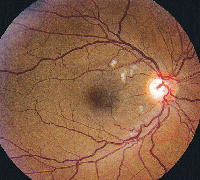
• Tamoxifen. Tamoxifen is a selective estrogen receptor modulator used in the management of breast cancer. It is an amphiphilic agent that can accumulate in lysosomes and cause oxidative damage.
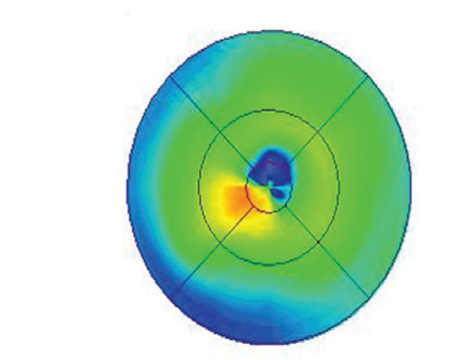
Clinically, fundus examination will show refractile intraretinal crystalline deposits concentrated primarily in the perifoveal macula (See Figure 2a). Both OCT and FA are used to confirm the presence of CME (See Figures 2b & c).
While the current therapeutic dosing levels are less than those shown to cause crystal deposition, annual ophthalmologic exams are recommended. Cessation of tamoxifen is recommended if crystals are seen. However, even if visual function recovers as the CME resolves, intraretinal crystalline deposits often persist over time.
• Canthaxanthine. Canthaxanthine is a vitamin A derivative used in the treatment of psoriasis and eczema, and had been used previously as an oral tanning agent. Toxicity has been shown after high-dose oral therapy of greater than 0.5 mg/kg/day.18 Patients are primarily asymptomatic and diagnosis of toxicity is based on both clinical exam and a history of ingestion.
On fundus exam, a doughnut-shaped ring of golden intraretinal deposits surrounds the fovea. FA, ERG and color vision are typically normal; however OCT demonstrates crystalline deposition within the inner retinal layers. Visual field testing shows a dose-dependent decrease in retinal sensitivity and may be helpful in detecting toxicity.
Patients should be monitored with regular ophthalmologic exams. With drug cessation, the retinal crystals may take years to disappear, while patients often remain asymptomatic.
• Talc. Talc retinopathy inevitably indicates a history of intravenous drug abuse. Talc, an inert filler used in oral powdered medications, deposits in the arterial system of the lungs and liver when injected systemically. It may also lodge in the macular arterial vasculature, causing a granulomatous reaction with focal occlusion, leading to eventual macular ischemia.19
The talc deposits range from 5 to 10 µm in size and are visualized as refractile yellow opacities in the macula. Ischemic sequelae include peripheral retinal or optic disk neovascularization, vitreous hemorrhage and arteriovenous anastomosis. Patients often present complaining of severe, progressive vision loss.
FA imaging will show capillary nonperfusion, enlargement of the foveal avascular zone and vascular leakage. Treatment of the peripheral neovascularization with panretinal photocoagulation is essential, however there is no effective treatment for macular ischemia once present.
Miscellaneous Medications
• Digoxin. Digoxin (Lanoxin) is a cardiac glycoside used in the treatment of atrial fibrillation, atrial flutter and congestive heart failure. It has a narrow therapeutic window, but systemic toxicity is rare when plasma digoxin concentration is less than 0.8 µg/L.
Ocular side effects are common with high doses, with symptoms ranging from decreased vision to photopsias, xanthopsia and scotomas. Digoxin acts on retinal cells by inhibiting the sodium-potassium ATPase pump and altering potassium levels. The disturbance is primarily electrical and fundus examination is unremarkable. ERG studies may show prolonged b-waves reflecting bipolar and Müller cell dysfunction.20
When digoxin is stopped, both the visual symptoms and prolonged ERG b-wave will resolve over weeks as the medication is metabolized.
• Sildenafil. Sildenafil (Viagra) is a selective inhibitor of cGMP-specific phosphodiesterase type 5 (PDE5) that causes smooth muscle relaxation and vasodilation in the treatment of erectile dysfunction. While it is selective for PDE5, sildenafil also acts on the retina to alter cGMP levels by inhibiting retina-specific PDE6.21
Patients may present with visual disturbances, complaining of cyanopsia, photophobia and blurred vision. Typically, visual acuity is unchanged and there are no fundoscopic changes on exam. Symptoms resolve over four to six hours after medication ingestion.
|
Recent case reports have also described nonarteritic, anterior ischemic optic neuropathy with sildenafil use.22 However, a recent review of pooled data from PDE5-inhibitor clinical trials did not find any direct evidence of an increased risk of NAION.23 It is still advisable, however, to inform patients of the need to seek immediate medical attention if sudden vision loss develops.
Retinopathy is a well-characterized side effect of treatment and includes cotton-wool spots, intraretinal and pre-retinal hemorrhage, and macular edema (See Figure 3). A wide incidence range of 18 percent to 86 percent has been reported for interferon retinopathy.24 Signs develop within two weeks to three months of treatment onset; patients may complain of blurred vision or be entirely asymptomatic. Vision loss, though rare, can occur and be irreversible. Patients with diabetes mellitus and/or hypertension are more likely to show clinical signs of interferon retinopathy and must be followed closely.
Clinical findings usually resolve spontaneously when interferon is discontinued, however the benefit of completing treatment must be weighed against the risk of permanent vision loss and discussed with the prescribing physician. At baseline, a full fundoscopic exam with photos is helpful to monitor the patient both pre- and post-treatment.
We have summarized above several classes of medications, as well as individual agents, that cause both ocular and retinal toxicity. The specific constellation of signs and symptoms for each medication is important to recognize in order to identify and detect toxicity, especially as many adverse effects are reversible with medication cessation.
Dr. Abbasi is an ophthalmology resident at the Kresge Eye Institute, Wayne State University Department of Ophthalmology. Dr. Tewari is a vitreoretinal surgeon at Kresge and is an assistant professor in the same department. Contact Dr. Tewari at the Kresge Eye Institute, 4717 St. Antoine Blvd., Detroit, Mich., 48201. Phone: (313) 993-0871, fax: (313) 577-2905, e-mail:
atewari@med.wayne.edu.
1. Mavrikakis I, Sfikakis PP, Mavrikakis E, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: A reappraisal. Ophthalmology 2003;110:1321-6.
2. Semmer AE, Lee MS, Harrison AR, Olsen TW. Hydroxychloroquine retinopathy screening. Br J Ophthalmol 2008;92:1653-5.
3. Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: A report by the American Academy of Ophthalmology. Ophthalmology 2002;109:1377-82.
4. Borodoker N, Del Priore LV, De A Carvalho C, Yannuzzi LA. Retinopathy as a result of long-term thioridazine. Arch Ophthalmol 2002;120:994-5.
5. Haimovici R, D"Amico DJ, Gragoudas ES, Sokol S. Deferoxamine Retinopathy Study Group. The expanded clinical spectrum of deferoxamine retinopathy. Ophthalmology 2002;109:164-171.
6. Rhee DJ, Goldberg MJ, Parrish RK. Bilateral angle-closure glaucoma and ciliary body swelling from topiramate. Arch Ophthalmol 2001;119:1721-3.
7. Sankar PS, Pasquale LR, Groskreutz CL. Uveal effusion and secondary angle-closure glaucoma associated with topiramate use. Arch Ophthalmol 2001;119:1210-11.
8. Grinbaum A, Ashkenazi I, Avni I, Blumenthal. Transient myopia following metronidazole treatment for Trichomonas vaginalis. JAMA 1992;267:511-2.
9. Postel EA, Assalian A, Epstein DL. Drug-induced transient myopia and angle-closure glaucoma associated with supraciliary choroidal effusions. Am J Ophthalmol 1996;122: 110-2.
10. Moroi SE, Gottfredsdottir MS, Schteingart MT, Elner SG, et al. Cystoid macular edema associated with latanoprost therapy in a case series of patients with glaucoma and ocular hypertension. Ophthalmology 1999;106:1024-9.
11. Mackool RJ, Muldoon T, Fortier A, Nelson D. Epinephrine-induced cystoid macular edema in aphakic eyes. Arch Ophthalmol 1977;95:791-3.
12. Jampol LM. Niacin maculopathy. Ophthalmology 1988;95:1704-5.
13. Coluciello M. Vision loss due to macular edema induced by rosiglitazone treatment of diabetes mellitus. Arch Ophthalmol 2005;123:1273-5.
14. Ryan EW, Han DP, Ramsay RC, Cantrill HL, et al. Diabetic macular edema associated with glitazone use. Retina 2006;26:562-70.
15. Perkovich BT, Meyers SM. Systemic factors affecting diabetic macular edema. Am J Ophthalmol 1988;105:211-2.
16. Tatti P, Arrigoni F, Longobardi A, Costanza F, et al. Retrospective analysis of rosiglitazone and macular oedema in patients with type 2 diabetes mellitus. Clin Drug Investig 2008;28:327-32.
17. Drenser K, Sarraf D, Jain A, Small KW. Crystalline retinopathies. Surv Ophthalmol 2006;51:535-49.
18. Espaillat A, Aeillo LP, et al. Canthaxanthine retinopathy. Arch Ophthalmol 1999;117::412-3.
19. Shah VA, Cassell M, Poulose A, Sabates NR. Talc retinopathy. Ophthalmology 2008;115:755.
20.Vivo RP, Krim SR, et al. Digoxin: Current use and approach to toxicity. Am J Med Sci 2008;336:423-8.
21. Marmor MF, Kessler R. Sildenafil (Viagra) and ophthalmology. Surv Ophthalmol 1999;44:153-162.
22. Egan RA, Fraunfelder, FW. Viagra and anterior ischemic optic neuropathy. Arch Ophthalmol 2005;123:709-10.
23. Laties AM. Vision disorders and phosphodiesterase type 5 inhibitors: A review of the evidence to date. Drug Saf 2009;32:1-18.
24. Hayasaka S, Nagaki Y, Matsumoto M, Sato S. Interferon associated retinopathy. Br J Ophthalmol 1998;82:323-5.