The company’s patent-pending IV Free MKO Melt (midazolam, ketamine and ondansetron) compounded, conscious-sedation formulation is an alternative option to IV anesthetic that is administered sublingually to sedate patients undergoing ocular and other surgical procedures. The MKO Melt, in troche format, provides consistent and predictable dosing and allows for quick and easy administration, resulting in increased positive experiences for patients and staff. Traditionally, sedation medications for ocular surgery have been administered intravenously, which requires IV medications and supplies, and additional staff to assist in preparation, administration and monitoring related to this process; all of these factors often cause delays and disruptions in the operating room. The formulations or variations thereof have been used in more than 1,000 LASIK and cataract surgeries to date as part of the investigators’ evaluation process, and a growing number of physicians have made the switch to IV Free conscious sedation, which is now available for $25.00 per two-troche pack. For more information visit IVFree.com.
The company also introduced its new Pred-Moxi-Nepafenac (prednisolone acetate, moxifloxacin hydrocholoride and nepafenac) combination topical eye drop formulation. Imprimis now offers four unique proprietary antibiotic, steroid and nonsteroidal combination LessDrops topical formulations: Pred-Moxi; Pred-Ketor; Pred-Moxi-Ketor; and the new Pred-Moxi-Nepafenac for use following cataract, LASIK, photorefractive keratectomy and other ocular surgeries. For information, visit godropless.com or lessdrops.com.
TearScience LipiScan Debuts
 |
TearScience, manufacturer of Lipi-View II and LipiFlow for the treatment of meibomian gland dysfunction, announced the release of LipiScan, the only dedicated high-definition gland imager that allows eye care professionals to efficiently evaluate meibomian glands in busy practices.
The company says LipiScan harnesses patented dynamic meibomian imaging technology to produce high-definition images of meibomian glands. LipiScan allows physicians to assess meibomian gland structure during routine workups in any practice setting.
In the past year, TearScience says it has significantly adjusted prices of LipiView II and LipiFlow equipment and treatment activators (disposables). The introduction of LipiScan will allow busy practices to efficiently integrate assessment of meibomian glands and do so at an affordable price. For information, visit tearscience.com.
BVI Offers FLACS Cannula to Manage Femto Incisions
Beaver Visitec International announced the global launch of a new, multi-functional cannula, designed to improve surgical efficiency and optimize clinical outcomes during femto laser-assisted cataract surgery. The primary function of the Visitec FLACS Cannula is to ensure all types of femto incisions (side-port, main and arcuate) are opened smoothly, easily and cleanly. The cannula’s leading edge is blunt to safeguard against stretching, distortion or creation of a second tunnel. It is also very thin, allowing it to glide through and open femto incisions with minimal effort, BVI says.
In addition, the cannula can be used to inject viscoelastic and/or other solutions into the eye, thereby reducing the number of instrument passes into the eye and hand-offs between surgeon and scrub nurse. As a multifunctional tool, use of the Visitec FLACS Cannula may decrease procedure time, potentially leading to increased room turn over and more efficient patient flow.
Because it is a single-use device supplied in a manufacturer-sealed sterile package, the cannula removes the risk of cross-contamination associated with reusable instruments and eliminates the need for costly and time-consuming sterilization protocols.
The Visitec FLACS Cannula complements other single-use product offerings from BVI that can be used during FLACS, including the 27G Visitec Hydrodissection cannulae and blunt Capsulorhexis Forceps. For information, visit
beaver-visitec.com.
iCare Tonometer: IOP Precision
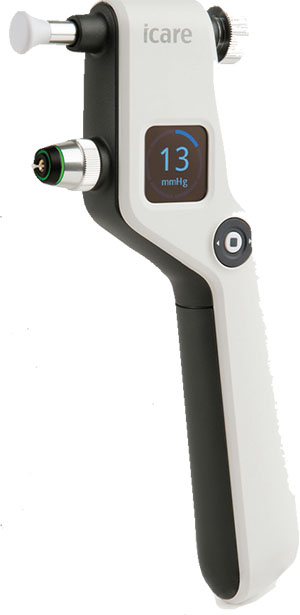 |
Icare USA has released the Icare ic100 tonometer. The Icare ic100 uses the same rebound technology as its predecessor, the Icare TA01i, with added ergonomic features and a user interface that takes intraocular pressure measuring to a new level, the company says.
The Icare EasyNav interface and the unit’s large color screen make this device exceptionally easy to use. It requires no calibration and is suitable for use on all patient types in virtually any setting. And, like its predecessor, the Icare ic100 requires no drops, air or specialized skills for use.
A built-in intelligent position assistant, called the Icare EasyPos, makes the Icare ic100 easier to use than ever before. Red and green lights help operators guide the tonometer into the correct position for testing. Examiners simply load, align and measure.
The unit features Icare AMS, an automated measuring sequence that takes a series of six measurements with a single touch of a button. For information, visit icare-usa.com.
ArcScan Precision Ultrasound For AC Measurement
ArcScan announced Food and Drug Administration 510(k) clearance for its ArcScan Insight 100 precision ultrasound device for imaging and biometry of the eye. Indicated for refractive surgical planning and evaluation of anterior segment pathology, the Insight 100 images and measures anterior chamber depth, angle-to-angle width, individual corneal layers, sulcus-to-sulcus width and more—with micron-level precision.
Unlike hand-held ultrasound, the Insight 100 allows users to easily obtain reproducible images with stunningly high resolution, the company reports. The ArcScan Insight 100 delivers visualization of the anterior segment’s true anatomy, including areas such as behind the iris in ways that optical technologies can not. Epithelial thickness mapping, one of its many applications, enables surgeons to confidently perform LASIK on patients they might have otherwise rejected because of suspect topography, and exclude candidates with early keratoconus who have normal-looking topography on less-advanced imaging technology.
To learn more about the ArcScan Insight 100 or to schedule a demonstration, visit arcscan.com; email info@arcscan.com, or call 1 (877) 363-7226. REVIEW



