 |
• The Folden Femto Dissector is a single instrument designed for smooth opening of all femtosecond laser-created corneal incisions during cataract surgery. Developed in coordination with David Folden, MD, the double-ended instrument measures 0.7 mm at one end and 1.2 mm at the other.
The polished, semi-blunted leading tip allows for “scoring” of the epithelium and provides easy, glided entry into the femtosecond laser-created corneal incision. The unique sharp-edge design cleanly separates residual tissue bridges and stromal adhesions that provide resistance to entry using standard instruments.
For clear corneal incisions, the 1.2-mm end provides easy entry into standard small incisions as well as sub-2.0 mm micro-incisions. The 0.7-mm end provides adequate clearance for entry into the paracentesis. Arcuate incisions for astigmatism are opened quickly and cleanly down to their base without risk of perforation. The polished semi-blunted leading tip glides smoothly along the base of the arcuate incision, while the sharp edge provides smooth opening of stromal tissue bridges and maintains clean epithelial edges. Fewer surface abrasions results in less foreign body sensation and improved patient comfort postoperatively, the company says.
• The Younger 360 Degree Capsule Polisher features a special angulated shaft that allows quick and easy polishing of both anterior and posterior capsules with one instrument.
Rhein says the unique angulation eliminates the need for using two instruments or using one instrument but having to exit and enter through a side-port incision to complete the polishing. The polisher is reusable, autoclaveable, U.S.-made and available for a 30-day surgical evaluation without obligation. Call (727) 209-2244 for more information on either product.
Konan Debuts Military-Grade Color Vision Test App
Konan Medical has released ColorDx Pro, a military-grade, extended color-vision diagnostics app for iPad.
 |
ColorDx is routinely used at the Naval Aerospace Medical Institute for qualifying naval aviators and the FAA has recommended its use for qualifying civil aviation pilots. ColorDx has other test strategies for vocational color vision assessment that are also self-administered and automatically scored.
ColorDx apps test for both genetic (protan/deutan) and acquired (tritan) color-vision deficiencies and are used extensively in clinical practices to aid decision making for a variety of neurological and ocular disorders, or substance toxicities that can cause a tritan deficiency.
ColorDx is also available for Android tablets, Windows and Mac computers as well as online and print. For information, visit konanmedical.com/colordx.
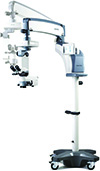
Coburn’s HOM-700 Surgical Microscope Streamlines Flow
Coburn Technologies introduces its latest addition to its diagnostic product line, the HOM-700 Surgical Microscope. Coburn says the HOM-700 streamlines all surgical workflow and maximizes surgery efficiency. This state-of-the-art product has a smooth system handling and superior performance with an optical system that is unequaled. It has high-resolution imaging for improved visuals and enhanced ergonomics to improve comfort and performance.
Among its key features:
 |
• fatigue-free surgery with its 10 x 21 mm visual field;
• an optimized halogen illumination system that reduces shadowing in deep cavities, provides high-quality illumination and prevents heating and UV transmission;
• optimized red reflex allows instant and perfectly stable red reflex from the illuminator providing the optimum brightness and observation angle;
• foot pedal control for hands-free surgery;
• a built-in, rotatable 7-inch color TFT LCD control panel;
• two onboard lamps allow the lamp to be changed instantly without interruption;
• custom settings allow up to four surgeons’ individual parameters to be saved and recalled effortlessly; and
• it’s fully compatible with industry-leading peripheral lens systems.
For more information, visit coburntechnologies.com.
| Product Research News |
Study of Artificial Tear Reveals Positive Effect on Dry-Eye Subjects
With still just one Food and Drug Administration-approved treatment for dry eye, researchers in the field continue to generate novel artificial tears with the hope of relieving patient symptoms and lessening signs of the disease. To date, emulsions are the latest and most innovative generation of dry eye therapy. Anionic oil-in-water nanoemulsions, while excellent vehicles for lipophilic drugs, have a poor retention time on the ocular surface. Cationic oil-in-water emulsions extend the benefits of anionic oil-in-water nanoemulsions by taking advantage of the negatively charged ocular surface, thus improving the residence time of the drop.
Recently, a single-center, open-label study was conducted to evaluate the efficacy of Retaine ophthalmic emulsion, a novel preservative-free artificial tear option, in patients diagnosed with dry eye. Retaine (marketed outside the United States as Cationorm, Santen, Osaka, Japan) is a proprietary, cationic oil-in-water nanoemulsion technology with novel bioadhesive properties.
In this Phase IV, two-visit study, 42 moderate to severe dry-eye subjects received one to two drops of Retaine b.i.d. for two weeks. Subjects were dispensed a diary with which they were to score their symptoms prior to each self-administered instillation (morning and night).
Following two weeks of dosing, study subjects demonstrated improvements in both signs and symptoms. At visit two, subjects had significantly less corneal fluorescein staining in the superior, central and the corneal sum regions. In the superior region, the mean score decreased from 2.2 to 1.93 (p=0.002) from visit one to visit two; in the central region, the mean score decreased from 1.25 to 0.95 (p=0.017); and in the corneal sum region, the mean score decreased from 5.55 to 4.95 (p=0.011). For all regions combined (i.e., the score of both corneal and conjunctival fluorescein staining), the mean score decreased from 9.36 to 8.67 (p=0.038). The reduction in central staining is clinically relevant, as the central cornea is critical to visual function (Ousler III G, et al. IOVS 2007;48:ARVO E-Abstract 410), while the improvement in the sum of the corneal regions indicates a global treatment effect.1-4
Additionally, significant reductions were observed in three ocular symptoms. Reductions were observed in discomfort (2.55 on the first visit versus 1.95 on the second visit; p=0.0017); dryness (2.88 versus 2.02; p<0.001); and grittiness (1.40 versus 1.02; p=0.0217). Also of note, the overall reduction of all ocular symptoms (i.e., the scores for all five symptoms combined) was significant (8.71 on the first visit versus 6.67 on the second visit; p<0.001).
Tear-film instability is a key feature of dry eye, one traditionally assessed by tear-film breakup time. This study employed a video-based technology (OPI2.0, Ora Inc.) that standardizes the measurement of corneal exposure. Across the study population, corneal exposure was reduced by 40 percent on the second visit between the pre-dose and the post-dose time points (p=0.026), indicating that Retaine has the ability to provide immediate corneal coverage.
Dry-eye sufferers often complain of impaired visual function during everyday tasks like reading, using a computer and driving, and perhaps more specifically, while completing these activities at night. The impact of the study drug on visual function was assessed by testing corrected visual acuity degradation between blinks; the time at CVA (time to one-line loss of CVA) was 41-percent higher on the second visit than the first (p=0.0697). Improved visual function also correlated with improved quality of life. Study subjects reported a significant improvement in their ocular discomfort when they worked at a computer at night (1.67 on the first visit versus 1.38 on the second visit; p=0.044).
Quality of life scores also decreased for reading at night, eye sight issues, watching television at night and driving at night.
In this two-week study, Retaine offered relief from both the signs and symptoms of dry-eye sufferers. The reduction in corneal exposure and in corneal fluorescein staining, coupled with the symptomatic relief and improvements in subject quality of life, equates to a product that provides total dry-eye relief.
1. Lemp M. Advances in Understanding and Managing Dry Eye Disease. Am J Ophthalmol 2008;146:350-356.
2. Cohen S, Martin A, Sall K. Evaluation of clinical outcomes in patients with dry eye disease using lubricant eye drops containing polyethylene glycol or carboxymethylcellulose. Clin Ophthalmol 2014;8:157-64. doi: 10.2147/OPTH.S53822. Epub 2013 Dec 31.
3. Kaido M, Matsumoto Y, Shigeno Y, Ishida R, Dogru M, Tsubota K. Corneal fluorescein staining correlates with visual function in dry eye patients. Invest Ophthalmol Vis Sci 2011;52(13):9516-9522.
4. Ousler G III, et al. “An evaluation of Retaine ophthalmic emulsion in the management of tear film stability and ocular surface staining in patients diagnosed with dry eye.” Clinical Ophthalmology 9 (2015): 235-43. Web. 5 Feb. 2015.



