|
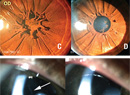
| Figure 1. “Light bulb” telangiectasias found in Coats’ Disease. |
The typical case is unilateral and progressive and affects males between the ages of 8 and 16 years. However, children as young as 3 weeks3 and adults4,5 have been reported to present with Coats’ disease. Females are certainly affected, though less frequently.6 Additionally, bilateral vascular abnormalities are found in about two-thirds of presumed unilateral disease, with peripheral non-perfusion >two disk diameters in contralateral eyes.7 There is a consensus that Coats’ is non-familial, but authors diverge on whether the condition is idiopathic or genetic. Mounting evidence suggests a causative mutation in the NDP gene encoding for norrin, found also in Norrie’s disease, resulting in abnormal retinal vasculogenesis.8 Microscopically this leads to dysfunctional pericytes and vascular endothelial cells, subsequent intra-retinal and sub-retinal serosanginous leakage, ischemia, aneurysms and progressively worse sequelae.
Early disease is often asymptomatic and diagnosed during routine examination. A classic finding is the loss of the normal red-reflex. It is replaced by a yellow reflex upon direct ophthalmoscopy and in flash photography as light reflects off of lipid exudation. The most common signs are decreased visual acuity, strabismus and leukocoria. Other signs include pain, nystagmus and heterochromia of the iris. On ophthalmoscopy, retinal telangiectasia, tortuosity, avascularity and aneurysmal dilation of retinal vasculature as well as vascular sheathing may be noted in early disease. More advanced disease may exhibit “light bulb” telangiectasia, named after large yellow exudates adjacent to dilated telangiectatic vessels (See Figures 1 & 2). Ultimately, this disease can progress to macular fibrosis, macular holes, retinal detachment and vitreous hemorrhage.
| ||||||||||||||||||||||||||||||||||||
|
Clinical Findings and Diagnosis
Clinical diagnosis based on history and exam alone is not usually sufficient, as other pathologies must be properly ruled out. Even the most experienced observer may not be able to distinguish between retinoblastoma and advanced Coats’ disease. There are some clinical clues that may help direct the clinician toward a diagnosis of Coats’, however. Familial exudative vitreoretinopathy (FEVR) may present with leukocoria and exudative retinal detachment, but usually is bilateral and associated with more tractional elements. Persistent fetal vasculature (PFV) may also present with leukocoria but lacks exudation, is often associated with central hyaloid canal and a smaller eye, and is usually accompanied by early cataract. Vasoproliferative tumors such as capillary hemangiomas and cavernous hemangiomas may also resemble Coats’, but there will usually be an absence of exudation and they occur more commonly in the peripapillary area, whereas Coats’ tends to affect the temporal macula and mid-periphery.
Computerized tomography is often used to rule out retinoblastoma, due to calcium content found in solid tumors but not classically present in Coats’. However, CT may miss nearly 50 percent of retinoblastomas that present without calcification,9 and conversely, Coats’ disease can present with intraocular bone formation. Magnetic resonance imaging with gadolinium contrast has been described as superior to CT in ruling out retinoblastoma10 due to increased contrast in enhancing solid tumors and in demonstrating subretinal exudation of Coats’. Fluorescein angiography is used for assessment of disease progression; early hyperfluorescence with patchy hypofluorescence from exudation can suggest telangiectasia, and “light bulb” dilations aneurysms can signal more advanced, larger vessel disease. Ultrasound is used to help rule out intraocular masses suggestive of retinoblastoma and reveal subretinal opacities representing exudates and retinal detachment from Coats’. Optical coherence tomography is used to detect macular edema, monitor response to treatment and even to perform intraoperative exam under anesthesia.11 Of note, fine-needle aspiration is not typically recommended if there is risk of retinoblastoma or retinal detachment, but may be potentially used as an adjunct when other testing is equivocal.
Prognosis
In an effort to more clearly describe risk factors for poor outcomes in Coats’ disease, Jerry Shields, MD, and coauthors proposed a five-stage classification system.12 In their retrospective consecutive study of 150 patients, the majority (76 percent of eyes) achieved anatomic improvement or stability, though poor final visual acuity between 20/200 and NLP occurred in 64 percent of eyes and enucleation was necessary in 16 percent of eyes. Significant risk factors for poor visual outcome were postequatorial, diffuse or superior pathology; residual subretinal fluid after treatment; retinal macrocysts; elevated intraocular pressure (over 22 mmHg); and iris neovascularization. Interestingly, age of onset of disease does not appear to correlate with final visual acuity.13 According to the Shields classification system (See Table 1), the incidence of poor visual outcome (equal to or worse than 20/200) was 0 percent in stage 1 eyes, but jumped to 53 percent in stage 2, 74 percent in stage 3 and 100 percent of stages 4 and 5.
 |
| Figure 2. Fluorescein angiogram in the late venous phase demonstrating peripheral “light bulb” aneurysms. |
Treatment
The Shields study also elucidated the optimal use of different treatment modalities based on stage of disease. The goal for mild disease (Stage 1 or 2) is prevention of retinal detachment, achieved by laser photoablation and cryotherapy for direct treatment of abnormal vasculature. Laser photocoagulation is preferred in mild cases with limited exudation; Amy C. Schefler, MD, and colleagues showed good results with this treatment modality in 50 percent of their patients14 and even demonstrated usefulness with subtotal retinal detachment. However, cryotherapy is preferred in mild15 cases with thicker exudates, in cases where laser photocoagulation is not available and in more advanced cases. Vitreoretinal surgery is typically required in cases of total retinal detachment or significant epiretinal membrane, while enucleation is performed in selected cases with neovascular glaucoma causing intractable pain, nausea and vomiting. There is ongoing research in cyclodiode treatment of these cases, with successful IOP lowering and avoidance of enucleation.16
Adjunctive therapy now includes intravitreal triamcinolone and anti-vascular endothelial growth factor medications. Intravitreal triamcinolone has been shown to help in absorption of subretinal fluid, reducing macular edema17 and exudates,18 and improving visual acuity.19 In a multitude of recent studies, anti-VEGF has also been shown to produce similar results.20-29 Anti-VEGF agents have a direct impact on vascular leakage and may yield resolution of Stage 3 and 4 disease. The physician must, however, be aware of the potential side effects of these agents, including infection, cataract and IOP elevation due to intravitreal triamcinolone. Emerging studies on the use of intravitreal dexamethasone implant (Ozurdex) as adjunctive treatment are promising.30 It must be remembered that few studies looked at these treatments in isolation from the mainstays of therapy: cryotherapy and laser photocoagulation. REVIEW
Dr. Chay is an ophthalmology resident and Dr. Shrier is a retina attending, both at SUNY Downstate Medical Center.
1. Shields JA, Shields CL, Honavar SG, Demirici H. Clinical variations and complications of Coats disease in 150 cases: The 2000 Sanford Gifford Memorial Lecture. Am J Ophthalmol 2001;131:561-571.
2. Ghorbanian S, Jaulim A, Chatziralli IP: Diagnosis and treatment of coats’ disease: A review of the literature. Ophthalmologica 2012;227:175-182.
3. Lim Fat CP, Lee SY, Brundler MA, Scott CM, Parulekar MV. Coats disease in a 3-week-old boy. J AAPOS 2014 Feb;18(1):86-8.
4. Andonegui J, Aranguren M, Berástegui L. Coats disease of adult onset. Arch Soc Esp Oftalmol 2008;83:117-120.
5. Smithen L, Brown G, Brucker A, Yannuzzi L, Klais C, Spaide R. Coats’ Disease Diagnosed in Adulthood. Ophthalmology 2005;112:1072-1078
6. Sigler E, Randolph J, Calzada J, Wilson M, Haik B. Current management of Coats disease. Surv Ophthalmol 2014Jan-Feb;59(1):30-46. doi: 10.1016/j.survophthal.2013.03.007. Epub 2013 Oct 15.
7. Blair MP, Ulrich JN, Elizabeth Hartnett M, Shapiro MJ. Peripheral retinal nonperfusion in fellow eyes in coats disease. Retina 2013;33:1694-1699.
8. Berger W, van de Pol D, Bächner D, Oerlemans F, Winkens H, Hameister H, Wieringa B, Hendriks W, Ropers HH. An animal model for Norrie disease (ND): Gene targeting of the mouse ND gene. Hum Mol Genet 1996;5:51-59.
9. Potter PD, Shields CL, Shields JA, Flanders AE. The role of magnetic resonance imaging in children with intraocular tumors and simulating lesions. Ophthalmology 1996;103:1774-1183.
10. Mafee MF, Goldberg MF, Cohen SB, Gotsis ED, Safran M, Chekuri L, Raofi B. Magnetic resonance imaging versus computed tomography of leukocoric eyes and use of in vitro proton magnetic resonance spectroscopy of retinoblastoma. Ophthalmology 1989;96:965-976.
11. Henry C, Berrocal A, Hess D, et al. Intraoperative spectral-domain optical coherence tomography in Coats’ disease. Ophthalmic Surg Lasers Imaging 2012;43:80-84.
12. Shields JA, Shields CL, Honavar SG, Demirci H, Cater J. Classification and management of Coats disease: The 2000 Proctor Lecture. Am J Ophthalmol 2001;131:572-583.
13. Budning AS, Heon E, Gallie H. Visual Prognosis of Coats Disease. J AAPOS 1998;2:356-359.
14. Schefler AC, Berrocal AM, Murray TG. Advanced Coats’ disease. Management with repetitive aggressive laser ablation therapy. Retina 2008;28:38-41.
15. Reichstein DA, Recchia FM. Coats disease and exudative retinopathy. Int Ophthalmol Clin 2011;51:93-112.
16. de Silva DJ, Brookes JL. Cyclodiode Treatment of neovascular glaucoma secondary to Coats’ disease. Br J Ophthalmol 2007;91:690-691.
17. Jarin RR, Teoh SC, Lim TH. Resolution of severe macular oedema in adult Coat’s syndrome with high-dose intravitreal triamcin olone acetonide. Eye 2006;20:163-165.
18. Othman IS, Moussa M, Bouhaimed M. Management of lipid exudates in Coats disease by adjuvant intravitreal triamcinolone: Effects and complications. Br J Ophthalmol 2010;94:606-610.
19. Bergstrom CS, Hubbard GB 3rd. Combination intravitreal triamcinolone injection and cryotherapy for exudative retinal detachments in severe Coats disease. Retina 2008;28:3337.
20. Stergiou PK, Symeonidis C, Dimitrakos SA. Coats’ disease: Treatment with intravitreal bevacizumab and laser photocoagulation. Acta Ophthalmol 2009;87:687-688.
21. Alvarez-Rivera LG, Abraham-Marín ML, Flores-Orta HJ, Mayorquín-Ruiz M, Cortés- Luna CF. Coat’s disease treated with bevacizumab (Avastin). Arch Soc Esp Oftalmol 2008;83:329-331.
22. Cackett P, Wong D, Cheung CM. Combined intravitreal bevacizumab and argon laser treatment for Coats’ disease. Acta Ophthal- mol 2010;88:48-49.
23. Cakir M, Cekiç O, Yilmaz OF. Combined intravitreal bevacizumab and triamcinolone injection in a child with Coats disease. J AAPOS 2008;12:309-311.
24. Entezari M, Ramezani A, Safavizadeh L, Bassirnia N. Resolution of macular edema in Coats’ disease with intravitreal bevacizumab. Indian J Ophthalmol 2010;58:80-82.
25. Kaul S, Uparkar M, Mody K, Walinjkar J, Ko-thari M, Natarajan S. Intravitreal anti-vascular endothelial growth factor agents as an adjunct in the management of Coats’ disease in children. Indian J Ophthalmol 2010;58:76-78.
26. Lin CJ, Hwang JF, Chen YT, Chen SN. The effect of intravitreal bevacizumab in the treatment of Coats disease in children. Retina 2010;30:617-622.
27. Wells JR, Hubbard GB 3rd. The effect of intravitreal bevacizumab in the treatment of Coats disease in children. Retina 2011;31:427-428.
28. Zhao T, Wang K, Ma Y, Jiang YR. Resolution of total retinal detachment in Coats’ disease with intravitreal injection of bevacizumab. Graefe’s Arch Clin Exp Ophthalmol 2011;249:1745-1746.
29). Ramasubramanian A, Shields CL. Bevacizumab for Coats’ disease with exudative retinal detachment and risk of vitreoretinal traction. Br J Ophthalmol 2012;96:356-9.
30. Martinex-Castillo S, Gallego-Pinazo R, Dolz-Marco, Marin-Lambies C, Diaz-Llopis M. Adult coats’ disease successfully managed with the dexamethasone intravitreal implant (ozurdex) combined with retinal photocoagulation. Case Rep Ophthalmol 2012;3:123-127.