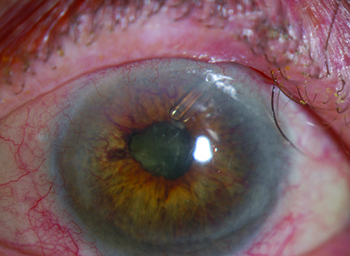
Potential causes of neovascular glaucoma include diabetic retinopathy; central retinal vein occlusion; branch retinal vein occlusion; ocular ischemic syndrome; tumors; chronic inflammation; chronic retinal detachment; and radiation retinopathy. (The most common causes are diabetes, CRVO and BRVO.) Retinal ischemia triggers the release of vasoproliferative factors, including vascular endothelial growth factor, fibroblast growth factor and interleukin-6. VEGF promotes the formation of fenestrations in new, immature vessels, allowing vascular hyperpermeability and increasing the level of inflammatory mediators in the eye; this may cause pain, independent of intraocular pressure. As vasoproliferative factors diffuse anteriorly from the retina to the anterior segment, fibrovascular proliferation in the angle causes obstruction of the trabecular meshwork and progressive synechial closure of the angle. The inevitable rise in IOP leads to neovascular glaucoma.
Because the impact of the disease evolves over time, it’s important to stage the disease when you encounter it. In stage 1, neovascularization occurs on the iris. You’ll see tufts of vessels on the anterior iris, usually at the pupillary margin. (Note: These may be difficult to see in dark irides.) Because the angle is unaffected, the IOP is normal at this stage of the disease. In stage 2, secondary glaucoma develops. Abnormal blood vessels now extend to the angle, causing fibro-blastic membranes to form there, blocking the trabecular meshwork—but without synechial closure. At this stage the angle still appears to be open, but intraocular pressure begins to rise. (Unfortunately, it tends to remain elevated, even if you achieve regression of the neovascularization.) Stage 3 is marked by secondary angle-closure glaucoma. The myofibroblasts formed by the abnormal blood vessels contract, leading to synechial closure of the angle and elevated IOP.
Key Factors to Address
When managing a patient with neovascular glaucoma you need to address at least four things that will affect whether the patient retains or loses vision: the presence of abnormal blood vessels; excessive VEGF factor inside the eye; inflammation; and (depending on the stage of the disease) elevated intraocular pressure. How you address these should depend, at least in part, on the stage of the disease when you encounter it.
• Regression of abnormal blood vessels. To address this part of the disease we inject intravitreal anti-VEGF therapy, usually bevacizumab (Avastin) or ranibizumab (Lucentis), although aflibercept (Eylea) and pegaptanib (Macugen) are also available. The neovascular growth will not disappear, but it will collapse within a few days to a week after injection as a result of diminished vascular permeability. The anti-VEGF agents also help by decreasing the pain associated with the inflammation that accompanies the disease, independent of making the blood vessels go away. (Unfortunately, these drugs only have a transient impact, so repeat injections may be necessary.)
These drugs are usually injected into the vitreous, although some surgeons have tried putting them into the anterior chamber angle as well, hoping to cause regression of the vessels blocking the angle. If the angle is not closed, this might allow the pressure to drop somewhat. However, most surgeons just place the drug intravitreally because these eyes usually have retinal ischemia.
• Addressing the ischemic drive to neovascularization and reducing inflammation. The former is best addressed via retinal ablation, whether it’s panretinal photocoagulation or cyclocryoablation. This will produce a sustained reduction of the ischemic drive that produces the vasoproliferative factors, along with a reduction in the amount of VEGF-producing tissue. Topical corticosteroids can be used to reduce inflammation.
• Lowering the elevated pressure. When treating neovascular glaucoma you also have to treat the elevated IOP. You can do this using medical therapy, including beta blockers, topical or oral carbonic anhydrase inhibitors, alpha-adrenergics or prostaglandin analogues. The prostaglandin analogues are equivocal because they can be pro-inflammatory, but they’re often used anyway, trying to prevent a bad situation from becoming worse. Atropine increases uveoscleral outflow and diminishes congestion, helping make the patient more comfortable. You should avoid anticholinergics such as pilocarpine because they can potentiate inflammation.
|
Another surgical option is to implant a seton, or tube shunt. Many surgeons choose this over trabeculectomy because it’s less affected by inflammation, which can cause closure of the trabeculectomy. Setons control IOP in 60 to 89 percent of these patients for the first year; however, the success rate diminishes to 10 to 46 percent at five years. (The type of tube used—e.g., Molteno, Baerveldt or Ahmed—doesn’t seem to affect the success rate.2)
The question then becomes, which surgery is best for treating neovascular glaucoma? The Tube vs. Trab study excluded patients with neovascular glaucoma; however the literature in general suggests that trabeculectomy is less likely to be successful, simply because inflammation is such a big factor in this disease.
This is even more important if the need to reduce pressure is urgent. I recently treated a patient who came in with neovascular glaucoma and a pressure of 70 mmHg. The eye was painful. In that situation, I couldn’t afford to wait until the abnormal blood vessels and inflammation had subsided. I had to address all concerns at the same time, so I chose a tube shunt rather than trabeculectomy. If you do have time to address the inflammation, then either surgery may be effective; if not, it makes the most sense to implant a tube shunt.
Staging and Visual Potential
The most effective treatment for neovascular glaucoma at any given point in time depends on the stage of the disease and the patient’s visual potential. The key thing to remember is the importance of treating the underlying disease.
If the patient is in stage 1—pre-glaucoma with abnormal blood vessels in the pupillary margin—you want to eliminate the neovascularization before the pressure goes up. Usually, that’s when you inject anti-VEGF drugs intravitreally. (Some surgeons have described cases in which they chose subconjunctival injection of bevacizumab, but most surgeons inject intravitreally.) You may want to do panretinal photocoagulation if there’s a lot of retinal ischemia. You can do goniophotocoagulation if you’re just trying to get rid of the abnormal blood vessels in the angle. You can also treat any inflammation that is present.
If the patient is at stage 2, the angle is open but the pressure is elevated because of all the abnormal blood vessels in the angle. In this situation you should do PRP and inject an anti-VEGF drug to decrease pain and complications. To address the elevated IOP you should provide one of the standard glaucoma treatments, whether it be in the form of eye drops or orally, and go on to glaucoma surgery if drugs can’t control the IOP.
By the time the patient gets to stage 3, where the angle is closed and IOP is elevated, you have to address all of these issues. So you want to do PRP, inject an anti-VEGF drug, reduce the inflammation and treat with glaucoma drugs and/or surgery. Usually, a glaucoma drainage implant is needed once the disease has progressed this far. In really bad cases, if the patient has very poor visual potential, you can use a cyclodestructive procedure to destroy the ciliary body. This helps to control the pressure in anywhere from 40 to 70 percent of these patients, but a lot of patients will end up with hypotony; phthisis is likely to occur in 2 percent of cases; and 22 percent will lose vision altogether. (Note: If you’re going to do a cyclodestructive procedure, whether it be laser or cryotherapy, preop treatment with an anti-VEGF drug will decrease the risk of a poor outcome.)
How aggressive you want to be when treating a patient with neovascular glaucoma depends on the level of vision the patient has. If the patient still has vision, you want to preserve it. So generally, you want to do the PRP and anti-VEGF to diminish vessel formation, and you want to do a trabeculectomy or implant a glaucoma drainage device to decrease IOP. If the patient’s vision isn’t good, but he’s not a good surgical candidate—perhaps a frail diabetic or someone on dialysis—you can do laser photocoagulation. If vision is limited, you can consider doing transcleral cyclophotocoagulation. If there’s absolutely no visual potential, at that point you go to comfort care. If the IOP is likely to remain high, you can make sure the patient won’t have pain as a result. You can use a retrobulbar injection to kill off the pain fibers in the optic nerve, or do enucleation or evisceration. At that point your job becomes to keep the eye comfortable.
Getting to the Cause
The most common mistake I’ve seen doctors make is not treating the cause of the problem. I’ve had patients whose previous doctor was focused on treating the elevated pressure, thinking he could address the neovascularization later. To preserve vision, it’s really important to start treating the cause of the neovascularization immediately, whether you treat with an anti-VEGF drug or with PRP. Especially if you’re planning to do glaucoma surgery, having neovascularization and inflammation will only make the surgery more difficult, and the outcome is more likely to be poor.
Even giving an anti-VEGF injection one day before surgery can make a huge difference; you’ll start seeing vessel regression within a day or two. Even if you catch the disease at stage 1, your results will be far better if you can cause the abnormal blood vessels to regress and eliminate the source of the VEGF, as well as any inflammation that’s already present. (Although anti-VEGF drugs are very helpful in the early treatment of NVG, PRP is still helpful for achieving long-term regression of vessels.)
I think most doctors encountering these patients do realize the nature of the problem, but if the IOP is already very high—say 60 or 70 mmHg—they may feel that addressing that pressure is their first priority, and rush to do surgery. In some situations, that may be appropriate, but the reality is that this is not like acute angle closure, where the pressure abruptly soars over a very short period of time. These patients have had their pressure increasing slowly as the angle became crowded, and in many cases the patient isn’t in much pain. In fact, when the pressure increases very slowly, it’s impressive how high it can get without the patient having much discomfort. If the patient had acute angle closure glaucoma and a pressure of 70 mmHg, she’d be throwing up and complaining of the pain. If your patient isn’t too uncomfortable, you and the patient may benefit from giving an anti-VEGF drug and other treatments a day or two to work before you perform surgery.
One other point: Some surgeons are concerned that an anti-VEGF injection may cause the IOP to rise a little bit. If you believe that’s an issue, create a small paracentesis to allow removal of a bit of aqueous when you put in the drug. That will help prevent the pressure from rising too high and threatening the optic nerve.
It’s Worth the Struggle
Unfortunately, even with IOP controlled, many studies have shown that anywhere from 3 to 48 percent of these patients will lose light perception.3,4 So there’s no question this is a tough condition to manage, especially in a profession in which so many of our patients have positive results. Nevertheless, appropriate treatment will indeed benefit these patients. Even though you won’t always win the battle, a lot of vision will be preserved. REVIEW
Dr. Huang is the founder of Seattle Ophthalmology. She has no financial ties to any product mentioned.
1. Brown GC, Magargal LE, et al. Neovascular glaucoma. Etiologic considerations. Ophthalmology. 1984;91:315–320.
2. Hong CH, Arosemena A, Zurakowski D, Ayyala RS. Glaucoma drainage devices: A systematic literature review and current controversies. Surv Ophthalmol 2005;50:1:48-60.
3. WuDunn D, Phan AD, et al. Clinical experience with the Baerveldt 250-mm2 implant. Ophthalmology 2006;113:766–772.
4. Mermoud A, Salmon JF, et al. Molteno tube implantation for neovascular glaucoma. Long-term results and factors influencing the outcome. Ophthalmology 1993;100:897–902.