Normal-tension glaucoma has much in common with high-pressure primary open-angle glaucoma, but there are some differences, both in terms of diagnosis and treatment, that should be considered. Here, I would like to offer some strategies to help clinicians manage these patients, in order to achieve to maximum preservation of vision.
Making the Diagnosis
Diagnosing normal-tension glaucoma is essentially the same as diagnosing primary open-angle glaucoma with elevated intraocular pressure; the difference is that the clinician is not alerted to the importance of carefully examining the optic nerve and anterior chamber angle by the red flag of elevated IOP. (By the time the patient reports symptoms, he has very advanced disease.) It is worth noting that any specific pressure chosen as the cutoff point between elevated and normal IOP is arbitrary.
Given the absence of the high-pressure warning flag, are there other signs that might alert us to the presence of glaucoma? Although there have been some reports of potential associations between normal-tension glaucoma and problems such as sleep apnea, migraine headaches or low blood pressure, it's not clear yet whether these are true risk factors; the statistical associations are fairly subtle. That means that if they are risk factors, they're not powerful risk factors. Certainly, we can't rely on them when screening for the disease.
Ultimately, there's only one truly effective and practical way to screen for normal-tension glaucoma among patients seeking comprehensive ophthalmic care: examining the optic nerve. (It is also essential to perform careful gonioscopy in every glaucoma patient and glaucoma suspect.) For this reason, it's crucial to dilate the pupil and look at the optic nerve using stereoscopic slit lamp biomicroscopy in every single patient. The direct ophthalmoscope used with an undilated pupil is great for certain things, such as looking for disc hemorrhages, but you can't rely on it for diagnosing glaucoma because you need to be able to evaluate the contour of the optic disc—something that can only be done accurately with a stereoscopic view. (Of course, a dilated examination may reveal other problems as well, so the potential benefits go beyond glaucoma.)
It's true that dilating the pupils takes more time and is less convenient for the patient, but it's the right thing to do, even for younger patients. Unfortunately, studies suggest that many ophthalmologists fail to regularly document the results of a dilated fundus examination in patients with a glaucoma diagnosis; I hope that these numbers at least partly reflect patients not wanting to be dilated, or simply missing appointments.
Once you've determined that a patient appears to have optic disc excavation or cupping, it's also important to consider whether something other than glaucoma might be responsible for the abnormality. One way to do this is to be cognizant of the clinical signs of non-glaucomatous optic neuropathies, which can sometimes cause the appearance of cupping. Their presence may indicate that a different process is taking place and needs to be addressed. 
An important sign that something besides glaucoma may be at work is optic disc pallor, which suggests the presence of an ischemic, compressive, hereditary or toxic optic neuropathy. Another sign is visual field loss that respects the vertical meridian, which indicates a chiasmal or retrochiasmal process. Thirdly, loss of visual acuity or an abnormality in color vision suggest the presence of a non-glaucomatous optic neuropathy (although they can occur in the setting of very severe glaucoma damage). Finally, the presence of a relative afferent pupillary defect may be an indicator of a non-glaucomatous process, although RAPDs can occur in the setting of asymmetric glaucoma damage. If a non-glaucomatous process is suspected, neuro-imaging or a neuro-ophthalmologic consultation should be considered.
Scans and Visual Fields
Many doctors use imaging instruments such as optical coherence tomographers, the Heidelberg Retinal Tomograph or the GDx Nerve Fiber Analyzer—which may help by drawing attention to optic nerve and nerve fiber layer abnormalities—or perimetry, to help them screen for normal-tension glaucoma. These tools may be of some benefit if you feel that you're not experienced enough to detect the disease with clinical examination of the optic nerve. However, all of these screening modalities have a major drawback: Their sensitivities and specificities are not ideal.
Visual field testing, for example, won't necessarily detect loss of function early on because of redundancy in the retinal ganglion cell system. Imaging devices are also limited because of variability of the optic nerve and RNFL in the normal population. So, if you rely exclusively on these approaches you'll pick up a lot of people who don't actually have a problem and miss some who do. That makes these instruments less than optimal for identifying the patients who need your help.
This is not to say that you shouldn't use them at all; if you believe you provide better care when screening patients with a visual field test or an imaging test, then go ahead. But the gold standard is clinical evaluation of the optic nerve using stereoscopic biomicroscopy.
Deciding Whether to Treat
In patients with elevated IOP and optic discs that are suspicious for glaucomatous excavation, I am more likely to begin ocular hypotensive therapy in the absence of a visual field abnormality than I am in patients with the same clinical findings but normal IOP. In contrast, if I suspect optic disc excavation in a patient with normal IOP, I generally do not initiate therapy in the absence of a glaucomatous visual field deficit. The exception to this is when I have photographic or imaging evidence that structural progression has taken place over time.
In the absence of a visual field abnormality or documentation of structural progression, one cannot be sure of the diagnosis of glaucoma; there is a lot of variability in the appearance of the optic nerve in the normal population. Some individuals have optic discs that are highly suggestive of glaucoma damage, but do not progress. So in this situation, I'd simply consider the patient a glaucoma suspect and monitor him more closely in the future.
Remember that unnecessary treatment is a burden for the patient: It's expensive; it causes discomfort; it may induce side effects; it reduces quality of life; and it forces the patient to think about the possibility of losing vision every day. If there is real disease, then the downsides to treatment are offset by the obvious benefit of a reduction in the risk of progression. But unless you're sure that you are treating a disease, you could be doing the patient a disservice. If you are not sure, it makes sense to monitor the patient and watch for solid evidence that the patient has glaucoma rather than an anomalous optic nerve.
The Role of Lowering Pressure
As with high-pressure POAG, the only known, effective, proven therapy for normal-tension glaucoma is IOP reduction, even though the IOP is normal at baseline. We know from two large, prospective clinical trials—the Collaborative Normal-Tension Glaucoma Study (CNTGS) and the Early Manifest Glaucoma Trial—that lowering IOP is beneficial and reduces the risk of progression in these patients.1-3
We believe that all primary open-angle glaucomas, including normal-tension glaucoma, have pressure-dependent and pressure-independent mechanisms. IOP reduction is beneficial in patients with normal-tension glaucoma because, even at normal levels, IOP appears to play a causative role in most patients. Therefore, further IOP reduction reduces the impact of the IOP-dependent causative mechanisms.
Genetic or environmental factors may make some people's nerve tissue more resilient or fragile than others'. That would help to explain how a pressure of 16 mmHg causes no problem for one person but is associated with optic nerve damage in a different individual. (It's true, of course, that some patients with normal tension glaucoma progress despite successful pressure reduction. At this point we don't fully understand the reasons, but this suggests that there are pressure-independent factors involved in the pathogenesis of the disease, at least in some patients.) At the same time, some individuals with elevated IOP don't develop glaucoma, which may indicate the presence of protective factors.
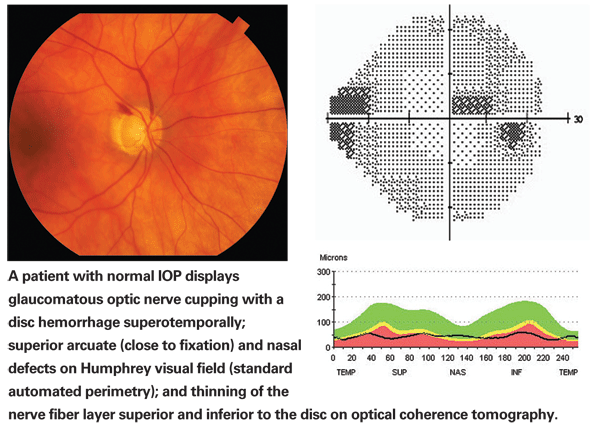
So, when I say that glaucoma is a multifactorial process, I mean that there are multiple factors that may lead to the development of glaucoma and multiple other factors that may protect and prevent the development of glaucoma, even in the presence of risk factors such as elevated IOP. Logically, it makes sense that in normal-tension glaucoma the pressure-independent factors would likely play a bigger role, and that might mean that some of the protective factors are missing in these individuals, allowing glaucoma to develop in spite of their normal IOP.
Hopefully, we will eventually understand all of the causal and protective factors that contribute to glaucoma (or its absence). Until then, we have to rely on the one treatment that has been proven to help most patients: lowering IOP.
How Much Reduction?
Based on the results of the CNTGS, in most cases we aim to reduce IOP about 30 percent once the diagnosis of normal-tension glaucoma has been made. However, deciding what amount of pressure reduction is acceptable depends on a number of considerations. How old is the patient? How is the patient's general health? How severe is the visual field loss, and is there a threat to fixation? How difficult will it be and what risks will be incurred in order to achieve a 30-percent reduction in IOP?
In the vast majority of NTG patients, I advise first-line medical or laser therapy. I reserve incisional surgery for patients in whom the target IOP is unachieved despite trabeculoplasty and maximally tolerated medical therapy. Suppose I have a patient with relatively mild normal-tension glaucoma and mild visual field loss. The patient is 80 years old, and in mediocre general health. If I can get the pressure down 30 percent, I'm certainly going to do it. However, if medical and laser therapy fail to achieve the target IOP, I would continue the medications that were effective and closely monitor the patient. I would avoid incisional surgery in such patients unless the disease progresses.
On the other hand, if I have a healthy 60-year-old patient who's progressing at a significant pace and I can't get the pressure down 30 percent using medications and laser trabeculoplasty, I would not hesitate to perform a trabeculectomy after a careful discussion of the risks and benefits. The potential benefits in that situation outweigh the risks.
Another consideration is that, as the Normal Tension Glaucoma Study demonstrated, about one-third of patients with typical glaucomatous optic disc excavation and typical visual field loss don't progress at all during an observation period of five years, even if they receive no therapy. So some patients with apparently significant vision loss when you first examine them will remain stable even without treatment. I'm not advocating that you observe all patients who present with this condition; after all, we don't know which patients will progress and which won't. In fact, I would treat the vast majority of these patients. But this does indicate the importance of taking into account the pace at which the disease seems to be progressing—if possible—when evaluating your therapeutic strategy.
Ultimately, the nuanced way of handling this situation is to consider each patient's situation individually. One should attempt to achieve the target pressure, advancing therapy as necessary, but keeping in mind that the risks and potential benefits must both be considered every time the therapeutic strategy is changed or intensified. In a patient with mild visual field loss and no evidence of recent progression, I would avoid incisional surgery even if a 30-percent reduction in IOP has not been achieved with medical and laser therapy. In fact, in such a patient, one may want to limit the number of medications used to just one or two.
Trabeculectomy is a good operation, but not a perfect one; some patients who have a trabeculectomy are going to lose vision as a result of the procedure. That level of risk should not be taken unless the likelihood of progressive vision loss from glaucoma is well-established. (Keep in mind that glaucoma progresses slowly, so in most cases we have the luxury of being able to monitor the patient before making this kind of decision.)
Generally, treatment should not begin at the first visit. The clinician should obtain at least two reliable and reproducible visual field tests after the patient has scaled the learning curve. The clinician should also make sure to have at least three baseline IOP measurements, preferably obtained at different times of the day, because IOPs vary depending on the time of day the measurement is taken, and from day to day. This will allow for a more meaningful determination of a target IOP. An additional consideration is that the presence of a disc hemorrhage indicates that the disease has a poorer prognosis; in this situation you may want to treat more aggressively.
Therapy Pros and Cons
Most commonly, the best first-line therapy for normal-tension glaucoma is the use of a prostaglandin analog or laser trabeculoplasty. The use of a nighttime beta-blocker is ill-advised. Beta blockers lower ocular perfusion pressure, and it's been shown in population-based studies that low ocular perfusion pressure is a risk factor for glaucoma prevalence. Moreover, some studies have found that use of a beta blocker at night in normal-tension glaucoma patients who were under treatment for systemic hypertension was an independent risk factor for visual field progression. So, in general, beta blockers should only be used once-daily, in the morning, in patients with NTG. (We are currently awaiting the results of a recent study comparing the efficacy of timolol b.i.d. and brimonidine 0.2% with respect to visual field stability in patients with NTG.)
Laser trabeculoplasty—either Selective Laser Trabeculoplasty or Argon Laser Trabeculoplasty—has been shown to be effective in patients with NTG. Studies in patients with high pressure POAG suggest that SLT and ALT are equally effective. As far as choosing between them, one could argue that there are theoretical advantages to SLT in terms of less tissue damage and possible repeatability, but the latter has not been proven.
If I felt that a patient would benefit from surgery—meaning that he was a good candidate and had not achieved a low enough pressure using medications and laser—I would perform a trabeculectomy. Of all the current incisional surgical approaches, trabeculectomy is most likely to achieve the low pressures that are generally required to treat this disease. (In my experience, newer surgical approaches like viscocanalostomy are less likely to result in the low IOPs usually required in eyes with NTG.)
Imperfect, but Workable
In the future, advances in our understanding of the causal and protective mechanisms involved in normal-tension glaucoma will undoubtedly increase our ability to successfully treat this disease. Certainly, neuroprotective approaches hold great potential, although attempts to date have not been fruitful. For now, even with our limited understanding and treatment options, most of these patients stand a good chance of avoiding vision loss. The key is catching the disease in its early stages—by dilating and examining the eyes of every patient—and making the most astute treatment choices.
Dr. Tanna is assistant professor of ophthalmology and director of the Glaucoma Service at the Feinberg School of Medicine at
1. Collaborative Normal-Tension Glaucoma Study Group: Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 1998;126:487-497.
2. Collaborative Normal-Tension Glaucoma Study Group: The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol 1998;126:495-505.
3. Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Arch Ophthalmol 2003;121:48-56.



