As a glaucoma specialist, it's important to have a sound relationship with a retinal surgeon. The older population we deal with often presents with retinal comorbidities, so our patients frequently overlap. In our practice we also end up doing many surgeries together; for example, patients with a corneal transplant may need a drainage tube because of secondary glaucoma caused by steroids or synechial angle closure. In that situation, anterior chamber placement of the device could compromise the graft, so I use a pars plana vitrectomy approach and enlist the help of one of our retina surgeons to perform the vitrectomy.
As part of that give and take, we frequently see retina patients following vitreoretinal surgery. Even in the case of a simple vitrectomy, approximately 40 percent of patients in one study were found to have a pressure greater than 30 mmHg in the immediate postop period.1 In addition, intraocular interventions performed at the time of surgery, such as intraoperative or previous scleral buckling, intraoperative scatter endophotocoagulation, intraoperative lensectomy and postoperative fibrin formation, may represent risk factors for pressure elevation after surgery. Surprisingly, the same study also found that a preexisting history of open-angle glaucoma did not increase the overall rate of IOP elevation after a vitrectomy. However, patients who had higher pressures acutely tended to have sustained high pressures at least six weeks after surgery.
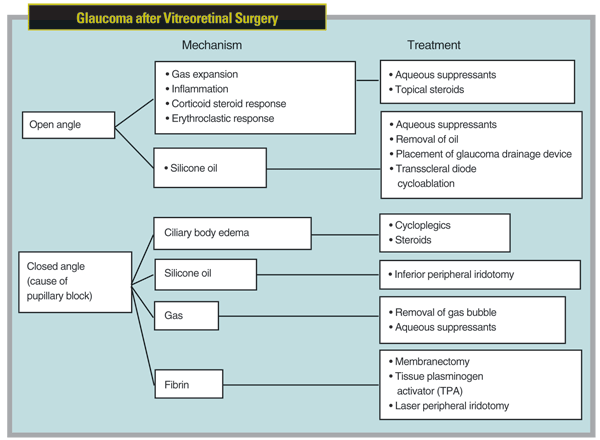
When these patients are referred to us, treatment for the elevated pressure should be based on the mechanism responsible for the elevated pressure, which may vary—even for one particular retinal procedure. For example, mechanisms may be closed- or open-angle. The possibilities for open-angle include gas expansion without angle closure—the most common cause of acute postvitrectomy IOP elevation, followed in descending order by inflammation, silicone oil (without pupillary block), corticosteroid response and erythroclastic glaucoma.1 The mechanisms for closed-angle glaucoma include ciliary body edema causing pupillary block, the most common mechanism, followed in descending order by pupillary block secondary to fibrin, gas, and lastly silicone oil. (For an overview of these possibilities, see chart, facing page.)
Fortunately, medical management can help address these postoperative pressure elevations and surgical intervention is infrequently required. Although one study reported the need for surgical intervention in 11.3 percent of subjects,1 another found that no patients required any surgical intervention.2 (When required, surgical interventions included anterior chamber paracentesis, laser peripheral iridotomy and laser iridoplasty or membranectomy.)
Complications of Scleral Buckles
The prevalence of primary open-angle glaucoma in patients with rhegmatogenous retinal detachments is 4 to 5.8 percent, compared to a 1.1 to 3 percent range in the general population.3-5 Several intraocular pressure issues can arise in the presence of a scleral buckle:
• Postoperative narrowing of the angle has been found in 14.4 percent of patients after the use of a scleral buckle.6
• In the same study, permanent abnormal intraocular pressure elevation and narrowing of the angle was seen in 3.75 percent of cases.
• The incidence of acute angle-closure glaucoma was 1.4 percent over a six-year period following scleral buckle.7
Risk factors for increased IOP after scleral buckle placement include: anterior placement of the buckle causing narrowing of the angle; older age; myopia; the duration of retinal detachment; and a history of previous narrow angles or choroidal detachments.6,8
In the presence of elevated IOP secondary to a closed-angle mechanism without pupillary block, the usual etiology is congestion of the ciliary body as a result of the buckle impeding venous outflow, causing engorgement of the ciliary body. The engorgement can cause the ciliary body to rotate forward and narrow the angle.
Frequently, the angle closure spontaneously resolves in the weeks after surgery as the choroidal effusions and ciliary body edema resolve. Medical management with cycloplegics helps to relax the ciliary body muscle, allowing a shift in the lens-iris diaphragm posteriorly, alleviating obstruction of the angle. There's no pupillary block involved in this mechanism, so laser peripheral iridotomy is not an effective option. Laser iridoplasty, however, would be beneficial. Never give these patients pilocarpine—this can exacerbate the condition by contributing to the narrowing of the angle.
When we encounter a patient with a scleral buckle who doesn't respond to medical or laser therapy, our surgical options are often limited to glaucoma drainage devices.
Because these eyes have a history of prior ocular surgery, the conjunctiva is usually scarred enough to preclude the possibility of a trabeculectomy even with antimetabolites. A drainage device can be implanted in either of two ways: We can shunt the aqueous through a silicone tube to the fibrous capsule surrounding the scleral buckle, as described in a study by New York Eye and Ear Infirmary's Paul A. Sidoti, MD,9 which found IOP control (IOP ≥21 mmHg) in 85 percent of cases, with obstruction of the distal tube by fibrous tissue occurring in 38 percent of eyes. Or, we can insert a glaucoma drainage device with a plate (usually a Baerveldt implant or Ahmed Glaucoma Valve). In one study this produced effective results in 56 percent of subjects, with IOP >5 mmHg and ≥21 mmHg without medications, and an additional 44 percent with IOP >5 mmHg and ≥21 mmHg with medications.10
Elevated Pressure and Silicone Oil
Silicone oil is used mainly when managing complex retinal detachments. Advantages over a gas tamponade include not having the patient maintain a certain position following surgery, less-distorted intraoperative visualization for the surgeon for laser retinopexy, and better postop acuity for the patient. Reported rates of chronic elevated IOP following use of silicone oil vary enormously—from 2.2 percent to 56 percent.11-13
The Silicone Study Report4 analyzed the data from the first Silicone Study group and determined its relationship to IOP; it found a prevalence of elevated IOP between 6 and 9 percent at 36 months after silicone oil injection.14 Risk factors for developing high pressure after silicone oil include: silicone oil in the anterior chamber; preexisting glaucoma; aphakia; early postop pressure spike; trauma; diabetes; and postop neovascularization of the iris.
As with the other retinal surgical interventions, elevated IOP can occur via several mechanisms. You may find acute angle closure with or without pupillary block; open-angle glaucoma with emulsified or non-emulsified silicone oil in the anterior chamber; continuing rubeosis of the iris leading to secondary angle closure; or simple idiopathic open-angle glaucoma.
The incidence of acute angle closure with pupillary block has dropped dramatically since retinal surgeons have placed prophylactic inferior peripheral iridotomies. An iridotomy at the 12 o'clock position is not indicated because silicone has a specific gravity less than water; it floats above aqueous superiorly in the eye where it may obstruct a superior peripheral iridotomy. However, these iridotomies still may close spontaneously, which has been reported in 14 to 17.8 percent of cases.15,16 The other point worth remembering is that angle closure with pupillary block is more likely if the patient is aphakic, because there's no intact lens/zonule system to keep the silicone oil out of the anterior chamber.
At one time, I surmised that whenever silicone oil made it into the anterior chamber, elevated pressure would surely result; however, when I reviewed the literature on this topic recently, I discovered that this is not the case. One study found that 40 percent of patients with silicone oil in the anterior chamber developed glaucoma.17 Other studies have found that visible oil in the chamber isn't necessary for a patient to exhibit high IOP18; that glaucoma developed in only 10 percent of patients who had emulsified oil in the angle19; and in one study, only 12 percent of patients had emulsified silicone oil in the anterior chamber postoperatively, and none of them developed glaucoma.13
Therefore, elevated pressures may occur with or without oil in the anterior chamber.
Frequently, removal of the silicone oil may not be enough to resolve the underlying problem; for that reason it remains controversial as a way to control of glaucoma. One paper reported that removal of the emulsified oil didn't change the intraocular pressure in 91 percent of subjects.20 In another study, silicone oil removal and medications produced pressure control in only 25 percent of patients.15 Another group of researchers found that both patients who underwent silicone oil removal, with or without glaucoma surgery, and those who underwent glaucoma surgery alone experienced satisfactory IOP control.21 This study also demonstrated that in contrast to patients undergoing silicone oil removal alone, those who had silicone oil removal and glaucoma surgery had higher rates of hypotony. Interestingly, they also found that four out of five patients with uncontrolled IOP after silicone oil removal who then underwent glaucoma drainage device placement had successful control. (However, these conclusions were made based upon a small subset of non-randomized patients.)
Decisions to surgically manage glaucoma in these patients must be thoroughly discussed with the retinal specialist, because removal of silicone oil prematurely is associated with a re-detachment rate in 11 to 33 percent of eyes.15 If the retina surgeon feels that it's safe to remove the oil, then I would proceed with its removal and simultaneous placement of a glaucoma drainage device; glaucoma drainage implants can control the IOP in the majority of eyes after pars plana vitrectomy and silicone oil injection.22 If the retinal surgeon feels the oil needs to remain in the eye for a longer period and aqueous suppressants are not effective, then consider placing a glaucoma drainage implant in an inferior quadrant or performing transscleral diode cycloablation.
Managing Intravitreal Gas
Gas is generally used for superiorly located retinal tears; the buoyancy of the gas bubble allows a good retinal tamponade as long as the patient is carefully positioned and the surface tension formed between the gas bubble and the surrounding aqueous can successfully prevent an accumulation of subretinal fluid. Elevated IOP is directly related to the expansile property and final volume of the intraocular gas bubble.
The incidence of high IOP after pars plana vitrectomy and long-acting gas tamponade was established by a report that found a 43-percent incidence of pressures greater than 25 mmHg.23 These gases can remain in the eye for 10 to 14 days for sulfur hexafluoride and up to 55 to 65 days for perflouropropane.24 Another report found that 52 percent of patients experienced an elevation over 25 mmHg and 29 percent experienced an elevation over 30 mmHg in the first four to six hours after surgery.25
Risk factors that would predispose a patient to an acute increase in IOP after gas tamponade include the concentration of the gas; older age of the patient; postop fibrin in the anterior chamber; concurrent use of a scleral buckle; and use of intraoperative endophotocoagulation.1,23
Clearly, a potential issue is the expansive gas bubble causing iridocorneal apposition without pupillary block glaucoma, or angle closure with pupillary block, which would require removal of the gas bubble. However, an open-angle mechanism is also possible, which could lead the retinal surgeon to send such a patient to a glaucoma specialist. In this situation the rate of expansion of the gas exceeds the rate of fluid leaving the eye, causing the elevation in pressure. Medical treatment with aqueous suppressants should be initiated.
A few notes of caution when dealing with this type of patient. First, an expansile gas in the eye can alter scleral rigidity, so you should not measure IOP using a Schiötz or pneumatic tonometer; they may underestimate the pressure. Applanators like the Perkins or Goldmann are preferable, and the Tonopen also is acceptable—although it tends to underestimate IOP if the pressure is above 30 mmHg.
You should also remind the patient to avoid receiving any inhaled nitrous oxide anesthetic (such as during a dental visit), because the nitrous oxide will elute from the blood stream into any adjacent gas-filled areas and increase the volume in these spaces.
With a gas bubble in the eye, this can cause pressure to skyrocket as high as 70 mmHg, leading to retinal artery occlusion and ischemia. Generally, it's suggested that these patients do not increase their elevation by 2,500 ft or travel by air where changes in the cabin pressure can instantaneously cause severe elevation of intraocular pressure.26
Keeping an Eye Out
Of course, a few glaucoma patients may come into your office with retinal concerns that have not yet been treated. As noted earlier, POAG patients have a higher number of retinal detachments than the general population, so it's important to look for this by dilating the pupil and examining the posterior chamber. In most cases, the presence of a retinal detachment in a non-glaucomatous eye does not cause elevated pressure by itself—usually, the opposite, possibly because the detachment allows a posterior flow of the aqueous. However, pressure may be elevated in Schwartz syndrome. The current explanation for this exception is that the photoreceptor outer segments may clog the trabecular meshwork. In fact, we occasionally find that patients presenting with visual field abnormalities have a retinal detachment rather than glaucoma, so it's essential to check.
Dr. Jamil is in practice at Glaucoma Consultants Northwest.
1. Han DP, Lewis H, Lambrou FH, Mieler WF. Mechanisms of intraocular pressure elevation after pars plana vitrectomy. Ophthalmology 1989;96:1357-1362.
2. Desai UR, Alhalel AA, Schiffman RM, Campen TJ, Sundar G, and Muhich A. Intraocular pressure elevation after simple pars plana vitrectomy. Ophthalmology 1997;104:781-785.
3. Scott IU, Gedde SJ, Budenz DL,
4. Becker B. In discussion of: Smith JL. Retinal detachment and glaucoma. Trans Am Acad Ophthalmol Otolaryngol 1963;67:731-732.
5. Phelps CD,
6. Sebestyen JG, Schepens CL, Rosenthal ML. Retinal detachment and glaucoma. I Tonometric and gonioscopic study of 160 cases. Arch Ophthalmol 1962;67:736-745.
7. Perez RN, Phelps CD,
8. Krieger AE, Hodgkinson BJ,
9.
10. Scott IU, Gedde SJ, Budenz DL,
11. Cibis PA. Recent methods in the surgical treatment of retinal detachments: intravitreal procedures. Trans Ophthalmol Soc U K 1965;85:111-127.
12. De Corral LR, Cohen SB, Peyman GH. Effect of intravitreal silicone oil on intraocular pressure. Ophthalmic Surgery 1987;18:446-449.
13. Nguyen QH, Lloyd MA, Heuer DK, Baerveldt G, Minckler DS, Lean JS,
14. Barr CC, Lai MY, Lean JS, Linton KLP, Trese M, Abrams G, Ryan SJ, Azen SP, The Silicone Study Group. Postoperative intraocular pressure abnormalities in the silicone study. Ophthalmology 1993;100:1629-1635.
15. Honavar SG, Goyal M,
16. Federman JL, Schubert HD. Complications associated with the use of silicone oil in 150 eyes after retina-vitreous surgery. Ophthalmology 1988;95:870-876.
17. Haut J, Ullern M, Chermet M, Van Effenterre G. Complications of intraocular injections of silicone combined with vitrectomy. Ophthalmologica 1980;180:29-35.
18. Watzke RC. Silicone retinopiesis for retinal detachment. A long-term clinical evaluation. Arch Ophthalmol 1967;77:186-196.
19. Valone J, McCarthy M. Emulsified anterior chamber silicone oil and glaucoma. Ophthalmology 1994;101:1908-1912.
20. Moisseiev J, Barak A, Manaim T, Triester G. Removal of silicone oil in the management of glaucoma in eyes with emulsified silicone. Retina 1993;13:290-295.
21. Budenz DL, Taba KE, Feuer WJ, Eliezer R, Cousins S, Henderer J, Flynn HW. Surgical management of secondary glaucoma after pars plana vitrectomy and silicone oil injection for complex retinal detachment. Ophthalmology 2001;108:1628-1632.
22. Ishida K, Ahmed I, Netland PA. Ahmed Glaucoma Valve surgical outcomes in eyes with and without silicone oil endotamponade. J Glaucoma 2009;18:325-330.
23. Chen PP, Thompson JT. Risk factors for elevated intraocular pressure after the use of intraocular gases in vitreoretinal surgery. Ophthalmic Surg Lasers 1997;28:37-42.
24. Chang S. Intraocular gases. In: Ryan SJ, ed. Retina. vol. 3.
25. Mittra RA, Pollack JS, Dev S, Han DP, Mieler WF, Pulido JS, Connor TB. The use of topical aqueous suppressants in the prevention of postoperative intraocular pressure elevation after pars plana vitrectomy with long-acting gas tamponade. Ophthalmology 2000;107:588-592.
26. Mills MD, Devenyi RG, Lam WC, Berger AR, Beijer CD, Lam SR. An assessment of intraocular pressure rise in patients with gas-filled eyes during simulated air flight. Ophthalmology 2001;108:40-44.





