Here, I’d like to discuss the pros and cons of each method and share my protocol for their use when managing glaucoma.
Seeing It for Yourself
Using gonioscopy to look directly into the eye can provide many visual clues to what’s going on that can’t be picked up by digital imaging. Gonioscopy also allows real-time manipulation of the eye and observation of the results. Gonioscopy is direct, interactive and can easily observe 360 degrees of the angle if the surgeon desires. In fact, the first line of defense for a glaucoma specialist is usually examining the patient and then doing gonioscopy.
One advantage of performing gonioscopy is that you can do a dynamic evaluation of the angle by pushing gently on the eye, causing a narrow angle to open up. This allows you to look for areas where there is chronic angle closure, which is signified by peripheral anterior synechiae; those areas will not open up in response to the pressure. Being able to do this test gives you information you can’t garner from looking at a single cross-sectional image.
Gonioscopy can also provide visual information, including color, that will allow you to rule out other conditions that can cause an angle to appear closed, such as neovascular glaucoma. Because you’re looking directly at the angle, you can see any neovascular vessels. You can also identify other types of glaucoma such as angle-recession glaucoma, and you can look for pigment from pigmentary or pseudoexfoliation glaucoma, the latter of which is also often associated with angle-closure glaucoma.
Gonioscopy has some practical advantages compared to imaging. For example, if you’re in the clinic with the patient in the chair, gonioscopy only takes a short amount of time to quickly assess whether the patient is occludable (180 degrees or more of grade 1 or less by the Shaffer system). In contrast, to perform anterior segment scanning using OCT you need to have the device, which can cost $50,000 or more. That makes OCT cost-prohibitive in poorer countries—and maybe even in a regular doctor’s clinic. Gonioscopy also has the advantage of portability; it can be done anywhere a slit lamp is available. The instrumentation required for high-resolution imaging, of course, is far less portable.
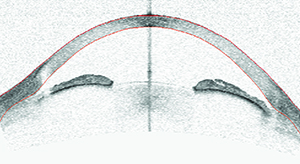 |
| Optical coherence tomography, ultrasound biometry and gonioscopy can each reveal things about the angle that cannot be detected using the other methods. Here, an eye judged to be occludable on gonioscopy shows narrow but open angles on OCT imaging. High lens vault and iris bowing make it difficult to see into the angle recess, resulting in the non-agreement with gonioscopy. |
The fact that gonioscopy requires a skilled surgeon also makes it a less-than-ideal choice for mass screenings. Having a doctor perform gonioscopy on a large group of individuals is time-consuming and expensive, especially compared to having a technician and a machine do the screening. If individuals are screened using OCT, those with a potential problem can later be identified by someone looking over the digital scans.
Despite the downsides, I believe every patient deserves to have gonioscopy performed, especially if the patient has glaucoma. Gonioscopy can help the doctor distinguish whether the problem is angle-closure glaucoma or open-angle glaucoma. The exam might also pick up other types of glaucoma, such as pigmentary glaucoma or pseudo-exfoliation glaucoma, and with some patients, it will help you rule out neovascularization in the angle. Gonioscopy provides many kinds of information that imaging can’t.
The Pros and Cons of Imaging
The best argument for imaging is that it’s a true cross-section of the angle that allows you to do quantitative measures of the angle, after identification of the scleral spur. The software also automatically gives you measurements of the angle recess area and the angle-opening distance, measurements that describe how narrow or open the angle is. That’s especially useful because cutoffs have been established regarding what constitutes an occludable angle that might require a laser iridotomy. Thus, imaging is quantitative in a way that gonioscopy is not.
Another argument in favor of using imaging to assess a narrow angle is that it’s very reproducible, which has been demonstrated in many studies.2 In contrast, gonioscopy is highly variable between different doctors—and even the same doctor can show some variability regarding whether the angle is grade 0, 1 or 2. Also, imaging can be performed by a technician. (And as the technology becomes more compact and portable over time, its use as a screening tool could become even easier and more common.)
One disadvantage of the kind of anterior segment imaging being done in the United States is that it only reveals one “slice” of the anterior segment at a time. While that is useful, it doesn’t tell us everything we might need to know
about the overall condition of the angle. This limitation may eventually disappear, however. There is now a device available outside the United States that can do 360-degree scanning of the angle: the Casia swept-source-1000 OCT from Tomey. This device quickly takes 128 scans as it rotates around the axis of the eye; then it constructs an accurate, measureable, three-dimensional model of the anterior segment. I’ve taken part in some studies using this device, but currently it’s only available in the United States in a few locations on an experimental basis.
| Whether OCT or UBM provides better digital information is another question that remains unanswered. |
Imaging with Ultrasound
The other type of imaging that’s useful when assessing the angle is ultrasound biomicroscopy. UBM imaging can reveal some things that anterior segment OCT can’t. For one thing, it can often help determine the mechanism of the glaucoma because it can penetrate through the iris and look at things behind it that may be causing the angle closure. It can be especially helpful when the problem is plateau iris, where the ciliary body is pressed up against the iris and pushing it forward. Anterior segment OCT can’t capture that information because it can’t penetrate through the iris. (Obviously, gonioscopy also can’t reveal what’s happening behind the iris.) This is important because plateau iris has been shown to be a major cause of angle-closure glaucoma; close to 20 percent of angle-closure glaucoma may be attributable to plateau iris.3
The major downside of UBM is the contact nature of it; it’s more cumbersome because it requires touching the eye. It’s true that the new UBM instruments are much less challenging for the patient than the old technology that used an eye cup filled with Goniosol jelly. But the probe still needs to touch the patient’s eye and the procedure still requires the use of an anesthetic; and of course you have to have well-trained technicians, because there’s a potential danger of scratching or injuring the cornea. In contrast, anterior segment OCT is noncontact and very quick.
Is One Approach Better?
As already noted, each of these methods for assessing the angle has benefits and drawbacks. Interestingly, they don’t always agree in their assessment. Studies have found that the agreement between gonioscopy and imaging is fairly poor. From an overall statistical viewpoint they correlate reasonably well; that is, if the doctor performs gonioscopy and says a patient is at risk of angle closure, the imaging will often agree. But when you look at specifics such as the grading of the angle, the correlation is poor. (The explanation for this poor correlation seems to depend on which method you favor. For example, people who really believe that scanning the angle gives the best representation of its condition would say that gonioscopy is the source of the problem because it’s so subjective.)
 |
| An iris cyst is revealed by ultrasound biomicroscopy. Such a cyst might not be observed on clinical exam and cannot be delineated using anterior segment OCT. |
My Protocol
So, what does a glaucoma specialist with experience in this area do? I believe my protocol is similar to that of most glaucoma specialists. I begin by examining the patient and then performing gonioscopy. Once I suspect that a patient may have a narrow angle, based on exam and gonioscopy, I do anterior segment OCT to confirm that diagnosis and decide whether the patient might meet the criteria for doing further treatment such as an iridotomy.
In most cases, I only resort to imaging with UBM if the patient still has a narrow angle after I’ve performed an iridotomy. That indicates a greater likelihood that the patient has plateau iris or another problem that only UBM would be able to detect, such as iris cysts. (For example, see facing page.) Many ophthalmologists think of iris cysts as being rare, but one study found that among younger angle-closure glaucoma patients, iris cysts were the number-two cause of angle-closure.4 My experience has confirmed that. For example, UBM revealed iris cysts in one of my younger patients; because she had a family history of glaucoma, I asked the first-degree family members to come in. All of the family members scanned had iris cysts that were causing angle closure.
It’s worth noting that not all ophthalmologists reserve UBM for special cases. Some of my colleagues in China don’t bother with gonioscopy at all; if a patient appears to be narrow based on the examination, they go straight to UBM. Many of them don’t have OCT because of the cost of the equipment. UBM is far less expensive.
Given the nature of gonioscopy and today’s imaging technology, I believe it’s important to have all of these options available. If you’re comfortable performing gonioscopy, it’s an excellent first option after examining a glaucoma patient or suspect. If you’re not comfortable
with it, anterior segment OCT will provide you with useful information; just bear in mind that it has limitations that may impact the accuracy of a diagnosis without additional information. REVIEW
Dr. Lin is a professor of clinical ophthalmology and director of glaucoma at the University of California, San Francisco. He has no financial interest in any of the products mentioned.
1. Hertzog LH, Albrecht KG, LaBree L, Lee PP. Glaucoma care and conformance with preferred practice patterns. Examination of the private, community-based ophthalmologist. Ophthalmology 1996;103:7:1009-13.
2. Smith SD, Singh K, Lin SC, Chen PP, Chen TC, Francis BA, Jampel HD. Evaluation of the anterior chamber angle in glaucoma: A report by the american academy of ophthalmology. Ophthalmology 2013;120:10:1985-97. doi: 10.1016/j.ophtha.2013.05.034. Epub 2013 Aug 23. Review.
3. He M, Friedman DS, Ge J, Huang W, Jin C, Lee PS, Khaw PT, Foster PJ. Laser peripheral iridotomy in primary angle-closure suspects: Biometric and gonioscopic outcomes: the Liwan Eye Study. Ophthalmology 2007;114:3:494-500. Epub 2006 Nov 21.
4. Ritch R, Chang BM, Liebmann JM. Angle closure in younger patients. Ophthalmology 2003;110:10:1880-9.