|
This is a good way to think about the drugs we prescribe, because we need to know not just the ways in which they can be friendly, but also the ways in which they can cause an adverse event. Steroids, in particular, have a long list of positive and negative effects. That makes it especially important to understand how they work in the eye, so you can make well-informed decisions when using them. Here, I’d like to review some of what we know about how steroids work, and why they are both friend and foe.
The Good and the Bad
The biological changes triggered by corticosteroids are extensive and complex. Generally speaking, a steroid receptor in the nucleus of each cell is stimulated by the drug, causing as many as 6,000 genes to be either expressed or suppressed—in other words, turned on or turned off—all within a few hours of exposure to the steroid. Adding to the complexity of the interaction, the biologic response will depend on the type of cell the steroid reaches. A keratocyte, for example, will respond to a steroid differently than a trabecular meshwork cell. Your job is to try to use the steroid to manipulate the genes and get the result you want to address the patient’s problem.
Steroids can have a number of positive effects:
• They can be anti-inflammatory. In the cell nucleus, steroid-activated glucocorticoid receptors attenuate the DNA-mediated release of pro-inflammatory cytokines and downregulate arachidonic acid. Glucocorticoids also reduce inflammation at the cellular level by inhibiting leukocytes—their concentration, migration and activity.
• They reduce vascular permeability. This reduces swelling.
• They inhibit phagocytosis and the release of growth factors. As a result, they inhibit wound healing and fibrosis.
• They inhibit fibroblasts, the cytokines that stimulate fibroblasts, macrophages and other factors that attract blood vessel growth. This helps to limit the wound-healing response.
• They reduce scarring. This is a primary reason steroids are used after filtration surgery. This effect is the result of multiple biochemical steps, including reducing the recruitment of monocytes and leukocytes.
For these reasons, among others, steroids help improve outcomes after trabeculectomy; they interrupt the wound-healing cycle, allowing filtration to occur. Today, we’re very dependent on steroids in glaucoma surgery, to help ensure that the sur-gery is successful.
Unfortunately, at the same time, steroids can do some counterproductive things:
• They can hasten the formation of posterior subcapsular cataracts. This is obviously a serious drawback if the patient is phakic.
• They can cause immunosuppression. As a result, for example, if someone uses a steroid drop for a long time, a fungal keratitis may develop.
• They can cause elevated intraocular pressure. Close to 35 percent of people have a worrisome rise in pressure with topical steroid use. This was studied by Mansour F. Armaly, MD, back in the 1960s. He put steroid drops in one eye of normal volunteers three times a day for a month. He found that there were three levels of responders: In 66 percent of the people the pressure went up by less than 5 mmHg. In about 30 percent, pressure increased by 6 to 15 mmHg. In about 5 percent the pressure went up more than 15 mmHg. These numbers suggest that you need to worry about one out of every three people in the healthy population having a steroid response.
Dr. Armaly also noted that in the low-response group, IOP increased for two weeks and leveled off. But in the intermediate and high-response groups, IOP continued to rise for four weeks—and we don’t know how long that rise continued. This suggests that checking IOP at two to four weeks may not be adequate to determine how high steroids will push the IOP in individuals who are intermediate- or high-level steroid responders.
What Causes the IOP Increase?
The problem of steroids causing an increase in IOP has been known to be an issue for about 65 years. Studies have demonstrated that the reason the pressure goes up when steroids are being used is increased resistance to outflow.1
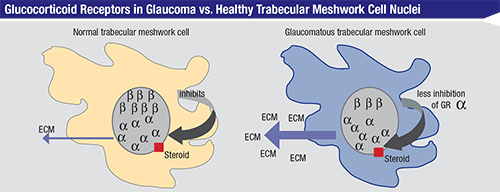
As noted earlier, generally speaking, a steroid receptor in the nucleus of each cell is stimulated by the drug. In a normal trabecular meshwork cell, the glucocorticoid receptors in the nucleus come in two types: alpha and beta. The beta receptor is believed to inhibit the alpha receptor, whose job is to manufacture extracellular matrix, or ECM, which can clog up your drainage system by forming a kind of soup around the cell. Normally the beta receptors modulate the degree of alpha expressivity, ensuring that the alpha receptors don’t make too much ECM, thus striking a balance that tends to maintain good outflow. However, in trabecular meshwork cells from glaucoma patients, there is an imbalance of the two receptors—a lack of expressivity of the beta cells. Therefore, the alpha receptors are left unchecked, leading to an excess of ECM that clogs up the outflow system.2 The bottom line is that the outflow system in patients who are high and moderate steroid responders becomes constipated. The pressure builds, and the patient ends up with steroid glaucoma.
It’s worth noting that steroid glaucoma and open-angle glaucoma look very similar. It’s certainly possible that non-steroid-related glaucoma has something to do with the alpha and beta receptors. Some people may simply be born with fewer beta receptors or receptors with less expressivity, making the person more susceptible to glaucoma because of excess ECM production by the less-inhibited alpha receptors. (This would also make these individuals more likely to be steroid responders.)
Steroids also have other negative effects on outflow that probably contribute to elevating the IOP:
• In addition to causing cells to make more ECM, steroids also inhibit the mechanisms that degrade ECM that help to keep the channels open. That results in increased accumulation of ECM and debris in the trabecular meshwork.
• Steroids cause cross-linking of actin, which alters the trabecular cytoskeleton. Cross-lin-ing the actin makes any blockage stronger and less permeable to flow.
• Steroids inhibit phagocytosis by trabecular meshwork cells, reducing the elimination of blockages.
• Steroids increase cell adhesion in the tight junctions. This may be the reason steroids inhibit blood vessel leakage, but you don’t want this in glaucoma. If the cells are more tightly bound together in the meshwork, they’re less likely to let fluid pass.
Of course, these changes are unlikely to lead to increased pressure if the patient has a functioning tube or trabeculectomy; those allow fluid to escape the eye regardless of any blockage in the trabecular meshwork.3
Other Considerations
Because the negative effects of steroids can be serious, it’s important to remember a few other things:
• Systemic absorption from topical drops is significant because it avoids first-pass hepatic metabolism normally seen with a pill. The steroid in topical drops is systemically absorbed through the nasal mucosa, just as happens with glaucoma drops such as beta blockers, allowing them into the bloodstream. One reason this is problematic is that it bypasses what is called “first-pass hepatic metabolism.” When you take a steroid in pill form, it goes into your gastrointestinal tract and ends up being partially detoxified by your liver before it enters the bloodstream. In contrast, when a drug is absorbed through the nasal mucosa, it’s like giving it intravenously. That’s why topical drops can have so many side effects; they’re not detoxified in their first pass through the body.
• Steroids in the vitreous can remain there for months. Kenalog or triamcinolone injected intravitreally can stay in the eye and have both positive and negative effects for months. They may provide significant help in addressing disease processes, but at the same time, they can make intraocular pressure increase.
• Once a steroid has been systemically absorbed, it can affect both eyes. No matter how a steroid is used in the eye or in the body, it will get into your bloodstream. One of the consequences of this is that using a topical steroid in one eye can cause an IOP rise in the fellow untreated eye.
|
Strategies for Success
Given the pros and cons of steroid use, it’s crucial to be on the lookout for trouble and take steps to protect your patient. These strategies can help:
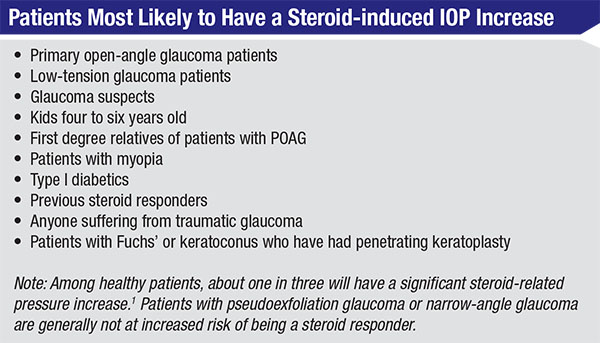
• Be aware of which patients are most likely to have a steroid-induced IOP increase. People falling into this category include:
— Glaucoma suspects, primary open-angle glaucoma patients and low-tension glaucoma patients. Studies show that if you have POAG, it’s almost a guarantee that steroids will cause your pressure to go up. Dr. Armaly found that 95 percent of POAG patients have a significant rise in pressure when treated with steroids. In one of his POAG studies, glaucoma patients who were off of their drops had an average IOP of 26 mmHg; with steroid drops, 50 percent of POAG patients had an IOP above 40 mmHg at two weeks.1 (I’ve seen pressure double in four or five days in a glaucoma patient on a q.i.d. steroid.) Likewise, almost 100 percent of low-tension glaucoma patients are steroid responders. Even glaucoma suspects have a much higher risk of a pressure rise than the general population.
— Kids 4 to 6 years old. They can have a significant pressure rise.
— First degree relatives of patients with POAG.
— Patients with myopia. This is especially likely in people with more than 5 D of myopia.
— Type I diabetics.
— Previous steroid responders.
— Anyone suffering from traumatic glaucoma. The reason for this is not yet clear.
— Patients with Fuchs’ dystrophy or keratoconus who have had penetrating keratoplasty.
Interestingly, if you have pseudo-exfoliation glaucoma or narrow-angle glaucoma, you’re not at increased risk of being a steroid responder. The reason isn’t clear, but whatever the genetic issue is in POAG patients, it doesn’t appear to be present in narrow-angle patients.
This list of likely steroid responders should always be in the back of your mind if you’re prescribing steroids for any reason.
• If a patient is on the list of likely steroid responders, adjust your treatment accordingly. Try prescribing a weaker steroid. For example, switch from Pred Forte to milder prednisolone acetate. Reduce the frequency of administration. Consider switching to an NSAID.
Also, check the patient’s pressure frequently. If you’re starting an at-risk patient on a steroid via any route—intravitreal, drops, periocular or oral—check IOP every two weeks, then monthly for two or three months; then every three to six months, depending on the type of risk factor the patient has.
• Consider having the patient use eyelid closure to minimize systemic steroid exposure from topical drops. When you put a drop in your eye and blink, that pumps some of the drug into your nose; from there, some is absorbed into your bloodstream. Simply keeping your eye closed for two or three minutes after putting in the drop prevents this from happening, increasing the absorption of the drug into your eye and decreasing the systemic absorption. Studies have shown that this can reduce systemic absorption by 60 percent.4,5
Another strategy people sometimes use to restrict the flow of drug into the nose is pressing a finger on the punctum. I don’t use this approach simply because I don’t like having patients put their fingers near their eyes. People don’t wash their hands that often, so this is riskier than simply closing the eye. Also, studies have found that nasal lacrimal occlusion and eyelid closure have similar success in reducing the systemic level of topically applied eye drops.5
It’s very common that the fellow eye’s pressure goes up after we do a filter. This almost certainly is the result of systemic absorption of the steroids used after surgery. If a patient has this problem, you may want to have the patient try eyelid closure; this simple strategy may bring the pressure back down in the other eye.
• If a problem persists and causes glaucomatous or optic neuropathy, consider glaucoma surgery. Trabeculectomy and tube surgery allow the fluid an escape that doesn’t depend on getting through a blocked trabecular meshwork. Most patients who don’t have preexisting glaucoma who get a pressure rise with steroids are very responsive to glaucoma drops. However, glaucoma patients who are already on several drops and then get a pressure rise in response to a steroid may need to have surgery in order to control the pressure.
• Be alert for systemic steroid problems caused by absorption of the topical drops. As already noted, a steroid drop placed on the eye will be at least partly absorbed systemically. In addition to possibly causing the pressure to rise in the fellow eye, systemic absorption can cause problems such as gastrointestinal ulcers. (I’ve seen this happen in patients taking a lot of steroid drops.)
• Be aware of the condition of the optic nerve. How much more pressure can it handle without further damage?
 |
• Remember that MIGS may not protect the patient from a steroid-related pressure rise. Minimally invasive glaucoma surgeries are getting a lot of attention these days. However, we’ve learned the hard way that MIGS won’t always protect you from a pressure rise related to steroids—unlike a trabeculectomy, where you’ve created an artificial external drain that keeps the pressure low.
Most MIGS surgeries, such as a stent or some kind of trabecular bypass or trabeculotomy, are designed to get the trabecular meshwork to flow better. If you use a lot of steroids in those people, the pressure can still go up. This may seem odd, but a recent study by Darryl R. Overby, PhD, may provide an explanation. We always used to think that steroids only clogged the upstream collector systems, like the trabecular meshwork. Now we’re learning that they can also clog things downstream, past the point at which you’ve opened up the outflow system with your MIGS. Dr. Overby’s study, using a mouse model, showed that fibroblasts in the downstream collector channels can turn into myofibroblasts, making excess ECM and clogging up the downstream collectors.6 The bottom line is that even though you’ve successfully done MIGS, it may not be as protective in steroid-responsive patients.
Striking a Balance
When it comes to prescribing drugs, the physician’s job is to balance the friend with the foe. This is particularly challenging with steroids. For this reason we have to be very careful when we give our patients steroids. Clearly we use them for specific positive purposes, but we have to make sure we find the best drug, the best concentration, the best frequency, the best route of delivery—which might be topical, peribulbar, intravitreal or systemic—and only have the patient use it as long as necessary. We have to know which patients are at the greatest risk of suffering an adverse effect from the steroid. We need to consider eyelid closure in susceptible patients to prevent the systemic side effects—especially pressure rise in the fellow eye. And we need to remember that having a glaucoma surgery such as MIGS is not an assurance that the patient won’t experience a rise in pressure from corticosteroids.
All of this is very different from our concerns when prescribing a typical glaucoma drop; there’s usually just one route to administer it. Of course, even glaucoma drops may occasionally become both friend and foe, causing cystoid macular edema, uveitis, follicular conjunctivitis, dry mouth, dry eye, hypotension, kidney stones, etc. But steroids are particularly potent as both friend and foe. Our practice in Dallas sees glaucoma patients exclusively, making us acutely aware of these issues. But the general ophthalmologist has an even tougher job because it’s more difficult to target the steroid responder while treating other diseases that may need steroid intervention. All eye-care providers need to keep the complete steroid picture in the back of their mind, to provide optimal patient care. REVIEW
Dr. Fellman is a clinical associate professor emeritus at the University of Texas Southwestern Medical Center in Dallas, and president of Glaucoma Associates of Texas. He has no financial conflicts with any product discussed in this article.
1. Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. The effect of dexamethasone in the normal eye. (I & II) Arch Ophthalmol 1963;70:482-99.
2. Clark AF. Basic sciences in clinical glaucoma: steroids, ocular hypertension, and glaucoma. J Glaucoma 1995;4:5:354-69.
3. Starita RJ, Fellman RL, Spaeth GL, Poryzees EM, Greenidge KC, Traverso CE. Short- and long-term effects of postoperative corticosteroids on trabeculectomy. Ophthalmology 1985;92:7:938-46.
4. Flach AJ. The importance of eyelid closure and nasolacrimal occlusion following the ocular instillation of topical glaucoma medications, and the need for the universal inclusion of one of these techniques in all patient treatments and clinical studies. Trans Am Ophthalmol Soc 2008;106:138-45.
5. Zimmerman TJ, Kooner KS, Kandarakis AS, Ziegler LP. Improving the therapeutic index of topically applied ocular drugs. Arch Ophthalmol 1984;102:4:551-3.
6. Overby DR, Clark AF. Animal models of glucocorticoid-induced glaucoma. Exp Eye Res 2015 Jun 4. pii: S0014-4835(15)00185-2. doi: 10.1016/j.exer.2015.06.002. [Epub ahead of print]