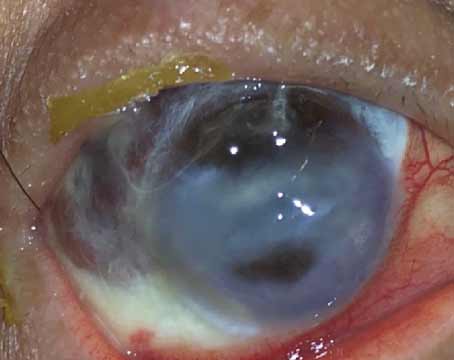
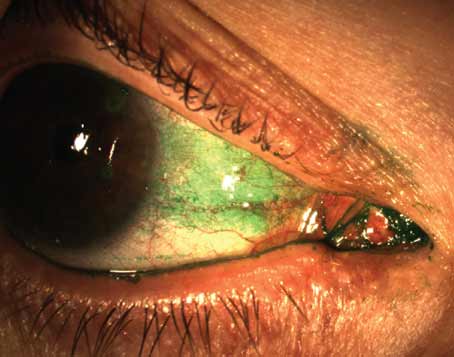
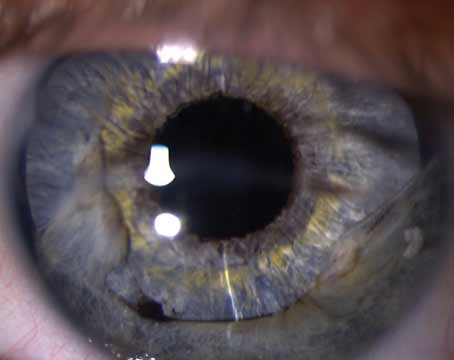
“Its usage is increasing and can be divided into two categories,” says Scheffer Tseng, MD, PhD, medical director of the Ocular Surface Center in Miami. “It can be used as a surgical graft, where the tissue is integrated into the host, and it can be used as a biological bandage. When used as a bandage, the tissue will not be integrated into the host. Instead, it will just be placed as a temporary dressing.”
When used as a graft, amniotic membrane provides the scaffold for re-epithelialization. “In other words, it is the foundation that skin can grow onto in cases where tissue has been lost for whatever reason,” says Darren Gregory, MD, an associate professor of ophthalmology at the University of Colorado School of Medicine. “It also provides an anti-inflammatory and anti-scarring effect that minimizes the buildup of scar tissue. The hope is to generate new tissue growth rather than just filling in the injured areas with scar tissue, because scar tissue can inhibit proper lid movement and movement of tears across the eye.”
The anti-inflammatory effect is especially important in cases of chemical burn or in patients with Stevens-Johnson syndrome. “In these cases, there is often intense inflammation that lasts two to three weeks,” Dr. Gregory says. “After it subsides, we can see that a lot of skin has been lost on the surface of the eye, and those areas fill in with scar tissue. Once that scarring has set in, it’s nearly impossible to get rid of and is a real challenge for patients and doctors.”
When used as a graft, AM is typically glued or sutured in place, and epithelium is expected to grow over it. When it is used as a bandage, the epithelium is expected to grow in underneath it rather than over the top. As healing progresses, the membrane gets sloughed off like a scab. When used as a bandage, it tends to degrade over the course of seven to 14 days.
Uses
“When amniotic membrane was first used in ophthalmology in the late ’90s and became commercially available, people were trying it for everything under the sun,” Dr. Gregory says. “I use it with some regularity because it does help with Stevens-Johnson syndrome. It is being used more and more in cases where there is a non-healing epithelial defect on the cornea, which can happen for a variety of reasons, but is usually either associated with corneal stem cell failure or a neurotrophic ulcer where the cornea has lost sensation because of a neurosurgical procedure, damage to the nerves from viruses, or a tumor that has damaged the trigeminal nerve, which provides sensation to the cornea.”
It has been shown to be successful in treating a variety of conditions. For example, in a study conducted in Italy with 12 years of follow-up, 5,349 surgical procedures were successfully performed using amniotic membrane patches.1 Conditions treated were corneal ulcers including neurotrophic keratitis (2,430); keratitis/endophthalmitis (363); pterygium (343); post-keratoplasty, glaucoma or cataract (339); chemical trauma (332); bullous keratopathy (265); neoplasm of the ocular surface (239); reconstruction of the conjunctiva and fornix (154); corneal degeneration (123); recurrent epithelial erosion (109); primary and secondary limbal stem cell deficiency (78); mucous membrane pemphigoid (59); dystrophy (58); mechanical trauma (25); chronic Stevens-Johnson syndrome or Lyell’s syndrome (20); reconstruction of the anophthalmic cavity (17); physical trauma (16); eyelid reconstruction (11); dysfunctional tear syndrome (9); and other (359).
The success or failure of the treatment was established with one year of postoperative follow-up. Success was determined based on the scope of surgery and the presence of one or more of the following criteria: resolution of inflammation; relief of symptoms; restoration of regular and stable corneal epithelium; and restoration of the structural integrity of the eye.
Partial success was defined as attainment of only two of the above criteria. Failure was defined as the absence of all of the above criteria. Conditions with a 100 percent success rate included: corneal ulcers including neurotrophic keratitis; post-keratoplasty, glaucoma or cataract; bullous keratopathy; corneal degeneration; dystrophy; mechanical trauma; reconstruction of the anophthalmic cavity; eyelid reconstruction; and dysfunctional tear syndrome.
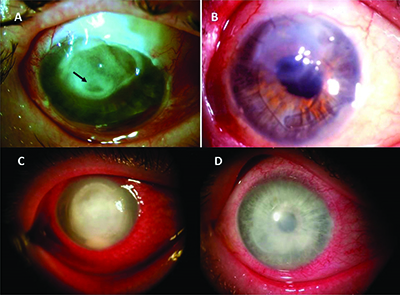 |
| Figure 1. Amniotic membrane can be used as a graft (A & C are before placement, and B & D are after) or bandage via Prokera. The top panel is an eye suffering from corneal ulcer and descemetocele (arrow) following a glaucoma shunt procedure and pseudomonas infection. After multiple layers of cryopreserved AM, the eye regained vision of 20/70 seven months later. The bottom panel is an eye developing infective keratitis following photorefractive keratectomy for myopia with a large corneal epithelial defect, stromal infiltration and hypopyon despite topical fortified antibiotics. Prokera was placed to reduce inflammation and promote healing to regain 20/50 vision in two months. (Image courtesy Scheffer Tseng, MD, PhD.) |
The study found that the therapeutic effects after treatment were variable and were related to the type of pathological condition treated. According to the study results, “The best results were obtained when the membrane was used to control inflammation and pain. In general, a higher success rate was attained when membrane transplantation was performed for its key therapeutic indication, i.e., persistent epithelial defect with stromal ulceration in patients with functional limbal stem cell deficiency. In such cases, the procedure was able to promote re-epithelialization in the majority of the patients and the restoration of a stable corneal epithelium in ulcers caused by different pathological conditions.”
The amniotic membrane was successfully incorporated into the corneal tissue in some patients, and membrane fragments remained visible for several days after surgery. In these cases, the membrane displayed its scaffold-like properties, as mentioned above, and provided a healthy and intact basal membrane on which the patient’s cells were able to proliferate.
Thomas John, MD, in practice in Chicago, says AM is now the standard of care for the treatment of acute Stevens-Johnson syndrome. Additionally, he has found that amniotic membrane in the treatment of acute toxic epidermal necrolysis preserves normal ocular and eyelid surfaces and may prevent blindness. Dr. John along with his co-authors published the first two cases of toxic epidermal necrolysis that were treated with amniotic membrane transplantation and described the surgical techniques involved.2 The first was a 6-year-old boy with severe toxic epidermal necrolysis that developed after being treated with trimethoprim and sulfamethoxazole for chronic otitis media. In this patient, both eyes and eyelids were affected. He underwent bilateral lysis of symblepharon and all adhesions. He then underwent bilateral amniotic membrane transplantation to the entire ocular surface, except the cornea. Because of the patient’s loss of eyelid skin, transplantation of amniotic membrane was performed on all four eyelids, and transplantation of strips of amniotic membrane at the eyelid margins was required. Thirteen years after bilateral ocular surgery, there was no symblepharon, good ocular surface wetting, and 20/20 uncorrected bilateral vision.
The second patient was an 8-year-old girl with severe toxic epidermal necrolysis associated with mycoplasma pneumonia. She had bilateral, diffuse keratoconjunctivitis, diffuse corneal epithelial defects and bilateral symblepharon. Bilateral amniotic membrane transplantation was performed using a symblepharon ring in the left eye. In this case, amniotic membrane transplantation protected both ocular surfaces and prevented conjunctival contracture without adhesion of the eyelids to the ocular surface. Central vision was preserved, and there was minimal peripheral corneal vascularization and mild conjunctival scarring of the tarsal conjunctival surface 34 months postoperatively.
“In these cases, if you don’t use amniotic membrane in the first 10 to 14 days, the eye can be potentially lost over time,” Dr. John says. “It also works well to treat corneal perforation and for other corneal restoration procedures, and it can be easily performed in most cases under topical anesthesia.”
However, it should be noted that, although it can be used to treat the conditions noted above, it is not ideal for all cases. According to Dr. Gregory, although AM is commonly used for pterygium surgery, he does not use it in primary cases of pterygium excision. The benefit of amniotic membrane in these patients is that it is a shorter procedure and is less technically demanding for the surgeon. The trade-off is that the recurrence rate is higher when AM is used.
 |
| Figure 2. Cryopreserved amniotic membrane covering the entire ocular surface in acute toxic epidermal necrolysis with severe eye inflammation and epithelial sloughing. |
Cryopresered vs. Dehydration
Several processing methods are used to preserve the membranes for in-office use. While fresh amniotic membrane has been shown to be effective in clinical applications, its use presents a significant risk of disease transmission. For this reason, processing methods that preserve its biological effectiveness while ensuring safety are important. One method is cryopreservation, which involves quickly freezing the tissue. It was “developed to maintain the structural integrity of the extracellular matrix and the endogenous biochemical functions of the native amniotic membrane and umbilical cord tissues.”4 In comparison, dehydration is a much harsher process and has been shown to cause protein denaturation, loss of function and irreparable damage to the ultrastructure and material properties of the tissue.
Dr. Tseng and his colleagues recently evaluated how these two different processing methods affect the structural integrity and biological composition of key signaling molecules within amniotic membrane and umbilical cord tissues.4 In this study, they directly compared cryopreserved amniotic membrane and umbilical cord tissues with dehydrated amniotic membrane/chorion tissue using biochemical and functional assays including histological and histochemical staining, bichinchoninic acid, agarose gel electrophoresis, western blot, ELISA and proliferation and cell death assays. The researchers found that cryopreservation retains the native architecture of the amniotic membrane/umbilical cord extracellular matrix and maintains the quantity and activity of key biological signals present in fresh amniotic membrane/umbilical cord, including high molecular weight hyaluronic acid, heavy chain-HA complex and pentraxin 3. The dehydrated tissues were found to be structurally compromised and almost completely lacked these crucial components.
Cryopreserved tissue requires refrigeration and has a limited shelf-life. “If it is in a bone freezer where it is -70°, it can be kept for up to two years, but some specialized freezers are required,” says Dr. Gregory. “Then, there is the freeze-dried or dehydrated form, which doesn’t require any specialized refrigeration. It can just be kept on the shelf and used as needed. You just rehydrate it. The concern with this form is whether it really retains all of the hyaluronic acid with a heavy side-chain protein that provides the anti-inflammatory effect. The sense is that the dehydrated form doesn’t retain as much anti-inflammatory effect.”
Dr. John calls the future of amniotic membrane transplantation bright. “In terms of the indications, amniotic membrane covers a huge area that encompasses the cornea, conjunctiva and the eyelids,” he says. “Patients, especially those being implanted with a premium intraocular lens, have high expectations, including a high quality of vision, fewer corneal aberrations and faster visual recovery. An optimal visual outcome depends on the ocular surface. Ocular surface health has really come to the forefront in ophthalmology in recent times, and amniotic membrane, especially an in-office Prokera type of device, is one way to augment and improve the ocular surface along with appropriate tear substitutes before performing cataract surgery with premium IOL or refractive surgery, especially in patients who have severe dry eye or ocular surface-compromising conditions.” REVIEW
Dr. Gregory has no financial interest in any companies that produce AM. Drs. Tseng and John have a financial interest in Bio-Tissue Inc.
1. Kushner BJ. The benefits, risks, and efficacy of strabismus surgery in adults. Optom Vis Sci 2014;91(5):e102-109.
2. Hopker LM, Zaupa PF, Lima Filho AA, et al. Bupivacaine and botulinum toxin to treat comitant strabismus. Arq Bras Oftalmol 2012;75(2):111-115.
3. Mireskandari K, Schofield J, Cotesta M, Stephens D, Kraft SP. Achieving postoperative target range increases success of strabismus surgery in adults: a case for adjustable sutures? Br J Ophthalmol May 19, 2015. [Epub ahead of print]
4. Leffler CT, Vaziri K, Cavuoto KM, et al. Strabismus surgery reoperation rates with adjustable and conventional sutures. Am J Ophthalmol 2015;160:385-390.