Current developments that are impacting the field include new ways to coax adult cells to return to a pluripotent stem-cell state; new ideas about using stem cells as a support network rather than replacements for diseased cells; and new insights regarding how to prevent unwanted side effects such as tumors. Here, three researchers share their latest work and discuss the state of stem cell research in general; where it may be in five years; and the potential benefits it holds for the field of ophthalmology.
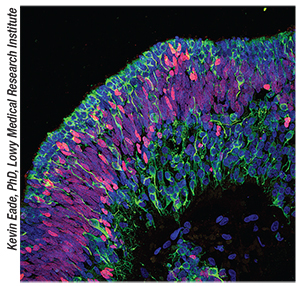 |
| A cross section showing mature human retinal neurons (green) derived from retinal progenitor cells (red) layered in an 18 week retinal cup. |
Martin Friedlander, MD, PhD, professor in the Department of Cell and Molecular Biology at the Scripps Research Institute in La Jolla, Calif., and chief of retina services in the division of ophthalmology at Scripps Clinic, along with members of his laboratory, have been at the forefront of several research projects that are shedding new light on a number of issues tied to stem cell use. One part of Dr. Friedlander’s work that could have a lasting effect on the field is the development of new ways to coax adult human cells into becoming induced pluripotent stem cells (iPS)—which can then be directed to become other types of cells—without using retroviruses.
The most significant advantage of using iPS cells instead of an alternative such as embryonic stem cells is that this method avoids the introduction of foreign cells into the recipient’s body, cells that the immune system might attack and reject. iPS cells have traditionally been generated from specialized adult cells via the introduction of retroviruses. Retroviruses, however, are believed to be the possible cause of developments such as tumor formation following the implantation of iPS cells. Dr. Friedlander’s group has found a potential way around this problem by getting specialized adult cells to become stem cells using small molecules instead of retroviruses.
“By using small molecules instead of retroviruses to get the cells to change, we avoid some of the potential oncogenes that can trigger tumor formation,” Dr. Friedlander explains. “We’ve been able to program the resulting stem cells to become retinal pigment epithelium cells, which we put into a rat model of RPE degeneration; the result was very good anatomic and functional rescue. That was exciting, not only because this method avoids a potential source of risk for the patient, but because the RPE cells that resulted were very similar to human fetal RPE cells. They shared a lot of characteristics both functionally and anatomically. We published a paper describing this work in 2012.”1
“In a more recent paper we asked the question, if you put these cells into the eye, how long do they remain effective?” he continues. “And are there any long-term potential downsides? To determine that, we’ve followed the progress of a number of the animals from the first experiment. At five years, the rescue effect had worn off, but there had been no adverse effects; the animals appeared to be perfectly fine and the grafts were still viable. In fact, we can recover the transplanted cells from the rats’ eyes after as long as four years.”2
Challenging Assumptions
Some of the work done by Dr. Friedlander’s team is calling into question common assumptions that underlie the hope that stem cells will provide simple and effective treatments. One discovery his team has made suggests that developing iPS cells from the patient’s own tissue may not always guarantee that the body will accept tissues developed from those cells.
“There’s a widespread belief that if you take autologous grafts such as skin or blood from the patient and derive iPS stem cells from those and then generate spare parts for the patient, such as RPE or heart or other organs, implanting them will not generate an immune response,” he says. “It makes intuitive sense that those parts, generated from the patient’s own cells, wouldn’t face the potential rejection issues that we may be facing with embryonic stem cell-derived spare parts, made from someone else’s cells. However, our work suggests that this may not always be true.
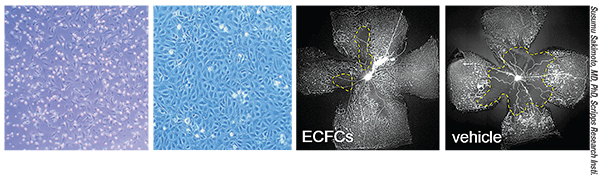 |
| Endothelial progenitor cells can be isolated from human cord blood and placed in xenofree culture media to grow as endothelial colony forming cells (far left). These cells, under appropriate conditions, will differentiate into endothelial cells (second from left) and can be used to normalize vascular defects observed in a rodent model of ischemic eye disease (right images). |
“It turned out that some cell types still did elicit an immune response,” he says. “Apparently, not all iPS-generated cells are created equal. Muscle tissue grafts, for example, triggered an immune response. However, the RPE cells that we generated did not.3 This suggests that generating a graft of some types of tissue from a patient’s own cells may not guarantee avoidance of rejection. On the other hand, it also provides good evidence that generating RPE cells in this way is likely to work.” Dr. Friedlander notes that this doesn’t have anything to do with the idea of the eye being immune-privileged. “A graft of RPE cells placed outside of the eye didn’t elicit an immune response either,” he says.
Another assumption Dr. Friedlander’s work is challenging is that all of the cells developed from a patient’s tissue will be identical. “If you want to recreate a patient’s disease in a dish using these cells as a way to test for disease manifestation, genetic abnormalities, functional abnormalities, response to drugs and so forth, it’s important that the cells not be different from the patient’s original cells,” he notes. “So, in collaboration with Kristin Baldwin’s lab here at Scripps, we ran an experiment involving what we call ‘sister clones.’ We used a patient’s blood or bone marrow to generate iPS cells and develop six different clones. We took one of those clones and used it to make subclones; we got six ‘daughter’ cell lines from that one clone. Then we checked to see whether all of the cells we derived were identical to one another. We found that the answer depended on the circumstances. Under some conditions they were identical; under other conditions they were not. In general, we did find that RPE derived from any of the clones looked virtually identical, if we gave them enough time to fully differentiate.
“The point is that generating tissue from the patient’s cells and then using that to help the patient is not as simple as it may sound,” he says. “So while I remain extraordinarily enthusiastic about the possibility of using autologous grafts—that is, tissues derived from a patient’s own blood or bone marrow or skin—I think we have a long way to go in terms of learning about how best to use these cells.”
Despite these issues, Dr. Friedlander says he still favors using the patient’s own iPS cells. “It makes sense because you don’t have to worry as much—at least in the case of RPE cells—about an immune response,” he says.
“However, we also have to consider the cost. It’s obviously going to be a lot more expensive to do individual cell-based therapies using the patient’s own cells than it will be to take an embryonic stem cell line and derive one RPE cell line that will fit many, many patients. But biologically, clinically and scientifically, the idea of individualized autologous cell therapy is a lot more appealing. Besides, many current therapies are even more expensive. Some people pay $50,000 for a year of treatment with Lucentis. Suppose you could tell a patient, ‘We’re going to use your tissue to make iPS cells and derive RPE cells from them and treat your Stargardt’s disease with one transplant every 10 or 20 years.’ Even if you charge $50,000 or $100,000, it’s still going to be cost-effective in comparison.”
| Regenerating the Crystalline Lens from Endogenous Stem Cells |
| One of the concepts gaining traction in stem cell research is the idea of activating existing stem cells in vivo, either by stimulating them to greater activity or by removing obstacles to their activation. The latter approach is epitomized by work being done at Sun Yat-sen University in Guangzhou, China. Researchers there announced in early March that they have been able to get naturally occurring stem cells inside the lens bag to regrow a crystalline lens in human babies, following the removal of a diseased lens. (The study appeared in the March 2016 online edition of Nature.4) Varying degrees of disorganized lens tissue regrowth have been observed after congenital cataract removal in infants; the researchers realized that the way the diseased lens is typically removed could be preventing a full lens regeneration. Currently, surgeons make a large 6-mm diameter capsulorhexis in the center of the anterior capsule to remove the lens, which prolongs recovery time and increases inflammation while eliminating most of the anterior subcapsular lens epithelial cells—including those needed for lens regeneration. In addition, abnormal proliferation of the remaining LECs commonly causes postoperative visual axis opacity that has to be addressed via posterior laser capsulotomy or capsulorhexis and anterior vitrectomy. All of this makes lens regeneration highly unlikely. To prevent such significant destruction of stem cells, the researchers developed a new way to perform the capsulorhexis. They created an opening 1 to 1.5 mm in diameter (about 4 percent of the size of a traditional capsulorhexis opening), and they made the opening in the periphery of the bag instead of the center. This reduced the incidence of inflammation, increased the speed of healing, improved postoperative visual axis transparency and preserved a nearly intact transparent lens capsule and layer of LECs. A study involving 24 eyes of 12 pediatric cataract patients, with a control group of 25 pediatric patients, found that the capsular openings healed within one month of surgery. At three months, a regenerated, transparent, biconvex lens structure of relatively uniform density had formed, and all eyes regained visual function. (No lenses formed in the group treated with the standard technique.) At eight months, the average central thickness of the new lens was comparable to a native lens. No significant visual axis opacity or other complications were observed at six months. The study authors note that lens epithelial cells in adult human eyes also show the potential for regeneration, although the regenerative capacity in adult eyes is undoubtedly diminished. In addition, phacoemulsification to remove hard cataracts in adult eyes could damage the cells, and tissue consistency and capsular thickness and elasticity issues may pose challenges as well. —CK |
One of the most interesting stem-cell-related developments in the past decade has been the realization that different organs contain cells that are partly specialized for use exclusively in that tissue, but still qualify as stem cells because their final cell type is not yet determined. Following that premise, researchers are now working with stem cells that are found in the RPE, as well as stem cells taken from the central nervous system, both of which are showing unique promise.
Researchers at the Neural Stem Cell Institute in Rensselaer, N.Y., have been working with the stem cells found in the retina. These cells are innately programmed to produce RPE cells (although they sometimes do produce other cells, possibly accounting for certain retinal disease conditions). Sally Temple, PhD, co-founder and scientific director of the institute, and president-elect of the International Society for Stem Cell Research, heads the group. They are currently culturing human RPE stem cells and transplanting them into diseased retinas in an attempt to produce anatomic and visual rescue.
“We now have really strong efficacy data for this procedure in an animal model using the Royal College of Surgeons rat,” says Dr. Temple. “In that model the RPE is dysfunctional and the photoreceptors die. We’ve shown that we can rescue photoreceptors and vision in that animal by implanting adult RPE stem cells. We’ve followed these animals for six months to date, and the effect is ongoing; the treated animals are continuing to see while the untreated controls are going blind.”
Dr. Temple says the cells are now being produced in a good-manufacturing-practice facility at the University of Rochester in New York. “We should have plenty for conducting future clinical trials,” she says. “We’ve done preliminary safety studies of our procedure, and they look good—there’s no evidence of abnormal growths or other problems. We’re now doing definitive efficacy studies, which will go into our IND application. A definitive safety study is planned for this year as well.”
One of the things Dr. Temple’s team discovered several years ago is that RPE stem cells sometimes turn into mesenchymal cells like those you would find in bone marrow—cartilage, fat and bone-forming cells. “That happens in some retinal disease states, such as epiretinal membrane formation, leading to retinal detachment and potential loss of vision,” she notes. “We’ve figured out some of the things that stimulate these stem cells to make that transformation, and we’ve also discovered some things that inhibit it. These discoveries are suggesting pathways and targets we can use to prevent this from occurring in the RPE cells we, or others, are transplanting. Perhaps even more important, they may help us prevent retinal problems like epiretinal membrane formation.
“This illustrates how stem cells are useful not only as a potential replacement for cells, but for disease-in-a-dish modeling,” she continues. “This approach will help us understand disease mechanisms and identify therapeutic agents. For example, we’ve shown that if we stress a culture of these stem cells in a dish using oxidation, we can detect drusen proteins—the kind of abnormal buildup of protein and lipid mixtures found in macular degeneration. So I think these cells will be useful for modeling that disease as well.”
Dr. Temple says her group is also working on finding ways to activate the endogenous stem cells already in the retina, which would have obvious advantages. “These cells are already in the eye,” she says. “If they can be activated in situ, then we would be able to avoid transplantation; we could simply revitalize those cells and have them regenerate and repair the RPE layer. That’s the next thing in the pipeline for us.”
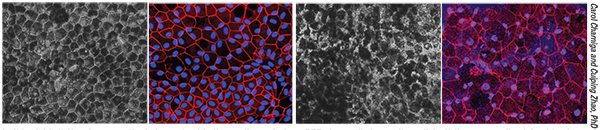 |
| Endothelial progenitor cells can be isolated from human cord blood and placed in xenofree culture media to grow as endothelial colony forming cells (far left). These cells, under appropriate conditions, will differentiate into endothelial cells (second from left) and can be used to normalize vascular defects observed in a rodent model of ischemic eye disease (right images). |
Another company pursuing the use of stem cells to support existing cells—rather than replacing diseased cells—is StemCells Inc. in Newark, Calif. The company is particularly interested in the potential of neural stem cells—unique cells found in the central nervous system that can differentiate into the building blocks of the central nervous system: astrocytes; neurons; and oligodendrocytes. Studies are indicating that an injection of these cells may slow the rate of progression of dry macular degeneration, and may even improve vision. (Banks of these cells, originally obtained from donated brain tissue, are now cryopreserved; they can be stored for years, and a small quantity can provide doses to a large number of subjects.) A unique advantage of this approach is that nothing needs to be done to change these cells; they can be harvested, grown and used without further alteration.
“Even though our cells are referred to as stem cells, they are not pluripotent,” explains Stephen Huhn, MD, FACS, a neurosurgeon by training, who is vice president and chief medical officer at StemCells Inc. “They can only become nervous system cells, so they don’t form tumors, which a pluripotent stem cell could do. In fact, that’s a risk that comes with transplanting a culture of RPE cells created from pluripotent stem cells, with the intention of replacing diseased cells. If you don’t transplant a pure population of RPE cells—if you have some embryonic or iPS cells mixed in—then you carry the risk of tumor formation. By injecting neural stem cells that we have not altered, we avoid this problem.”
Dr. Huhn says the company has performed a Phase I/II study involving patients with severe geographic atrophy. “We studied 15 subjects for a year,” he says. “We compared the treated eye to the fellow eye. What we found was first and foremost, safety; the patients tolerated both the procedure and a few months of mild immunosuppression that we used postoperatively. From an efficacy standpoint we saw some intriguing outcomes. Best-corrected visual acuity remained stable or improved slightly, and nine of the 15 patients had a noticeable improvement in contrast sensitivity from baseline. Other findings included that macular volume and foveal thickness, measured using optical coherence tomography, improved in the study eye compared to the fellow eye. Also, the progression of geographic atrophy was slower in the study eye in select patients.
“The data was good enough to warrant a Phase II trial, which we started last year,” he says. “However, we are a small biotech company, and financial concerns forced us to suspend the trial partway into the enrollment phase. We’re currently looking for a partner who can help us develop this potential treatment for dry macular degeneration. In the future, we hope to complete a controlled study that will demonstrate that this is a viable way to treat macular degeneration.”
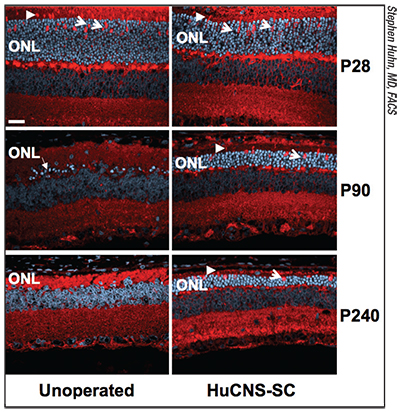 |
| Photoreceptor survival in the outer nuclear layer in a rat model of retinal degeneration, with transplanted human CNS stem cells (right column) compared to no transplant (left column), at days 28, 90 and 240 of the life cycle. The stem cells were transplanted at day 21, preserving the ONL layer out to more than seven months post-transplant. Arrows point to preserved cone photoreceptors. In the unoperated animals the ONL layer disappears to a few bright blue cells at Day 90 and is essentially absent at Day 240 |
One of the key issues this work has raised is what the stem cells are actually doing once they’ve been transplanted. “Over the years we’ve collected preclinical data relating to a number of neurological and neurodegenerative disorders, not just in the eye,” says Dr. Huhn. “One of the themes that we’ve seen in our preclinical data is the concept of neuroprotection. Rather than replacing diseased cells, the donor neural stem cells exhibit properties that support, rescue or protect neuronal populations. This could be the result of the stem cells releasing trophic factors, or other biological properties of the cells.
“Given that photoreceptors are sensory neurons, we wondered whether we could use our cells to protect the photoreceptors and stabilize vision in someone who was suffering a retinal degenerative disorder,” he continues. “Our studies produced very compelling animal data in a rat model showing that the neural stem cells would migrate within the subretinal space, graft to the retina and rescue vision out to seven months when we looked at two different visual-function measures. It was very robust data.”
Dr. Huhn says the company is also working to discover what the neural stem cells are actually doing that produces the positive effect. “One of the things we recognized in the animal study was that the debris field in the subretinal space was cleared in the areas where the cells engrafted,” he says. “Right away, that made us think that the neural stem cells may be taking over the phagocytosis activity that was formerly supported by the RPE, and we did find some evidence of that. The story is probably more complicated, however; I suspect some of the trophic factors and cytokines produced by the neural stem cells are exerting a neuroprotective effect on the photoreceptors as well. So even though we’re not replacing diseased cells, this treatment may stabilize them and possibly even improve their function.”
Dr. Huhn confirms that the neural stem cells do not appear to be turning into RPE cells. “We’ve seen migration of the cells into the inner retina, but we have not seen any cells that look like they’re becoming RPE cells in the animal models, even many months post-transplant,” he says. “We don’t see any shared RPE markers. The implanted cells tend to remain in the neural stem cell state, probably because the subretinal space doesn’t have the physiological signals that might direct the cells to grow into RPE cells.”
Dr. Huhn notes that there’s nothing wrong with the alternate strategy of trying to replace the diseased cells. “The problem is that replacing photoreceptors involves overcoming a whole set of really complicated biological hurdles,” he says. “You have to transplant a significant number of cells in a semi-mature, if not mature, differentiated state. Those cells don’t usually like to be transported, and they won’t migrate. Even after they’re in the right location they still have to integrate with the host circuitry of the retina. Our idea was that you might not need to do all those things to achieve a therapeutic benefit. If you can stabilize the vision of someone who has dry AMD with a one-time treatment, particularly early in the course of the disease, and have the protective effect last for years, then you can achieve a therapeutic result without the burden and complexity of replacing RPE cells or photoreceptors.
“Given that this treatment works by supporting the existing cells rather than replacing them, once we’ve shown that it can work for macular degeneration, there are many other eye disorders it might also help to treat,” he adds. “We believe this mechanism of action, in particular, will lend itself to multiple indications.”
| Steering Clear of Untested Treatments |
| Because the public is aware of stem cell research and the occasional advances that are reported in the media, individuals hoping for respite from a given condition may seek out stem cell treatment. Unfortunately, this has provided an opportunity for some people to offer treatments that are untested and perhaps even fraudulent. For a patient, the result can range from disappointment to serious health problems, on top of a potentially significant financial loss. Some reports have noted that this is going on in other countries, but Sally Temple, PhD, co-founder and scientific director of the Neural Stem Cell Institute in Rensselaer, N.Y., says it’s happening in the United States as well. “One of the challenges in the field right now is that some clinics are providing unproven therapies that claim to cure patients of all manner of diseases, including blindness or diabetes,” she says. “These clinics are charging people a lot of money for very questionable procedures. As a result, it’s important to get the word out about the right places to go for help, and what questions people should ask to be well-informed and protected from unproven or dangerous treatments.” Dr. Temple says that the International Society for Stem Cell Research has created a website called “A Closer Look at Stem Cells” (closerlookatstemcells.org) designed to help educate anyone interested in pursuing treatment. The website features downloadable resources such as the Patient Handbook on Stem Cell Therapies (available in 12 different languages); a booklet of facts about stem cells; a stem cell glossary; and a list of questions patients need to ask before proceeding with a stem cell treatment, among many other resources. “Prospective patients can visit this site and look at the questions they should be asking and learn how to investigate whether or not a purported stem cell therapy is really a good therapy,” Dr. Temple says. “This is one way the society hopes to protect patients from untested, expensive and potentially harmful treatments. |
In line with Dr. Huhn’s current thinking, Dr. Friedlander’s team wondered whether the improvement in vision seen after transplantation was caused by the transplanted RPE cells taking over the function of damaged or dead cells—i.e., replacing them—or by the new cells providing some kind of support for the existing cells. “We had reason to think the transplanted cells might be causing a trophic effect, because of the work we’d done with bone marrow and cord blood-derived progenitor cells,” he says. “We used a technology called targeted metabolomics, a cutting-edge version of mass spectrometry, to evaluate millions of molecules present in retinal samples taken from a rat with severe degeneration after a period of time with the stem cells in the eye.
“We worked with one of the world’s foremost authorities on metabolomic analysis, Gary Siuzdak here at Scripps,” he continues. “With his help we compared the metabolites in retinas from normal rats, rats with severe retinal degeneration and rats that had achieved vision rescue after being treated with our iPS-derived RPE cells. It turns out the most severely disregulated class of metabolites we could detect were fatty acid amides, which act as signals during normal metabolic activity. In particular, one specific fatty acid amide called erucamide was severely disregulated. Normal rats had high levels of erucamide, while rats with retinal degeneration had dramatically lower levels of erucamide. In the rats who had been rescued by the iPS-derived RPE cells, the levels of fatty acid amides and erucamide had been restored.
“Given this result, we wondered whether just injecting erucamide might have the same rescue effect,” he says. “In fact, we found that injecting erucamide did produce the same rescue effect. That’s the idea of trophic action. You put the stem cells in and they know what to do—they upregulate, or modulate the expression of many different molecules, some of which have very profound vascular and neurotrophic effects. I think a lot of the effect we see from people injecting stem cells is not because the stem cells are replacing the damaged tissue directly, but because the cells are providing a very profound trophic rescue effect because of their ability to upregulate molecules that are important for maintaining normal homeostasis.”
Dr. Friedlander notes that this supports the idea that early intervention is better. “If you put these cells in and they restore homeostasis before the retina is severely damaged, the outcome is likely to be a lot better,” he says. “That’s what I call ‘restoration rather than resurrection.’ ”
Dr. Friedlander says they’ve seen similar evidence in studies they’ve done involving macular telangiectasia. “Groups that have access to adaptive optics, like Austin Roorda’s group at UC Berkeley, Jacque Duncan’s group at UCSF and Joe Carroll and Alf Dubra at the Medical College of Wisconsin, have now shown that in patients where the photoreceptors appeared to be completely gone—because you couldn’t see them even with adaptive optics—the photoreceptors weren’t really dead and gone. Actually, the rods and cones had just lost their outer segments, while the inner segments remained. That means if you can figure out how to get those cells to re-sprout their outer segments, they could theoretically become functional again. That’s an absolute game-changer in terms of what we have to do to bring back vision in patients who have retinal degenerative diseases. We need to restore the cells rather than replace them. And that may be exactly what stem cells are doing.”
Asked whether the transplanted RPE stem cells her team is using are replacing diseased cells or simply providing support, Dr. Temple says in some cases they may be doing both. “There are cells in clinical trials now that we know cannot turn into RPE cells, such as umbilical cord cells,” she says. “In that case, when we see a positive result we know that the stem cells are not replacing the diseased cells per se, but are probably releasing trophic factors that are beneficial. In other cases, such as our research, or in the case of iPS-derived or embryonic-stem-cell-derived RPE, you’re generating new RPE cells, so they at least have the opportunity to replace some of the lost or damaged cells, in addition to releasing trophic factors.”
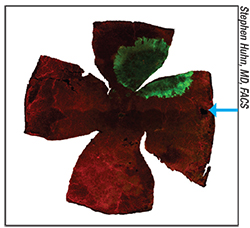 |
| A radial pattern of human CNS stem cell migration (green) in a rat retina at day 90 after transplantation. The blue arrow marks the spot at which the cells were injected. |
Since many approaches to implanting stem cells involve cells that did not originate with the recipient, use of some immunosuppression is common. Naturally, this raises concerns with many doctors.
The technique being used by StemCells Inc. involves a short course of low-level immunosuppression. “There are many schools of thought about this,” says Dr. Huhn. “Some believe that we shouldn’t need any immunosuppression at all. We don’t agree, because the blood-retinal barrier is disrupted for a period of time after you do the retinotomy and the vitrectomy. We have patients take an oral agent for a period of three months, and in our experience, patients tolerate this very well if they are generally healthy. In fact, our data indicate that after a period of immunosuppression is applied and then stopped, cell engraftment lasts at least six years.
“I think our approach to immunosuppression will become much more sophisticated and informed as the field evolves,” he adds. “But at this early stage, we think it’s better to include some course of immunosuppression as long as there are no safety concerns.”
Dr. Huhn admits that it might be possible to derive a neural stem cell from an iPS cell made from the patient’s own cells, possibly avoiding the need for immunosuppression. “However, that involves a lot of time and effort and expense,” he says. “Hopefully, that will not turn out to be necessary.”
Dr. Temple notes that because the RPE stem cells used in their protocol are not from the individual being treated, their current treatment protocol also includes a short period of immunosuppression. “When you cause bleeding during the surgery, you have blood cells invading that space,” she explains. “This could set off an immune reaction. Most current stem cell trials that use cells not taken from the patient are having the subjects undergo at least a short period of immunosuppression. The hope is that patients will only need to do this for a few months, until the effects of the surgery have healed. Hopefully, by then the blood-retina barrier will have recovered and the cells will not be rejected. Of course, we have to see if this is what actually happens.”
The Road Ahead
Dr. Huhn notes that the field of stem cell research is still largely in what might be called a “Phase I” stage of development. “I think the field has been largely focused on showing safety and tolerability up until now, and that may continue for a little while longer,” he says. “But people are beginning to think about trial designs for the more mature studies, so we’re kind of emerging from that phase now. The next thing I expect the field to deliver is proof of efficacy, in Phase II or III studies that are well-designed, that will convince everyone that there is merit to this kind of approach.
“If you think about it, in medicine we’ve had success with solid organ transplantation and bone marrow transplants for a long time,” he continues. “Today we can do partial face and hand transplants. So far, we haven’t been able to do that with the eye, brain or spinal cord. But with neural stem cells, we have the ability to go down that path. We know that the concept of transplantation works; it’s just a question of how we can dial it in for the retina and the eye.”
Dr. Temple says she’s been thinking a lot about the state of stem cell research in general because she’ll become president of the International Society for Stem Cell Research starting in June of this year. “There’s a lot of exciting research taking place,” she notes. “Understanding the process of human development and the production of retina, brain and other tissues is really important. Stem cells are giving us the opportunity to learn things that have been out of our reach until now. In the past we’ve been able to study the development of tissues in rats and mice, but we now know that more than 60 percent of gene expression in mice is different from humans. That means we need to have human RPE cells in the dish so that we can truly understand human-related mechanisms.
“Creating disease-in-a-dish models is burgeoning right now,” she continues. “For example, microcephaly has been in the news a lot because of the Zika virus, but it can occur through a number of different genetic perturbations. Now researchers are using little 3-D organites created in a dish from stem cells to study processes like microcephaly formation. Also, there’s a big push to screen small molecules to see if they can revert disease phenotypes and prevent the development of disease. I predict that’s going to become an even bigger focus of research as we get better disease models and streamline the process of integrating these methods with high-throughput screening.”
Dr. Temple notes that transplantation-based treatments are still the focus of a significant amount of stem cell research. “We’ve seen success with limbal cell transplants for many years now,” she points out. “There are many groups around the world working on RPE transplantations for macular degeneration, in Israel and Japan and the U.K. and here in the United States. If we can get this type of procedure working and approved, it will provide a revolutionary new treatment for macular degeneration. Meanwhile, the disease-in-a-dish model may allow us to create therapies for earlier-stage disease that won’t require a cell transplant and immunosuppression. We’ll be able to just use small molecules and prevent the RPE from degenerating.”
Other areas of stem cell research not directly involving the eye will also likely have a positive impact on ocular diseases. “Researchers at a company called ViaCyte who are working to manage diabetes have developed a device that incorporates pancreatic islet cells—the ones that normally make insulin—generated from pluripotent stem cells,” says Dr. Temple. “In this device the cells are shielded from the immune system, but they’ll be able to respond to the levels of glucose in the bloodstream and produce insulin accordingly. It’s going to be much more responsive than the typical insulin injection regimen. This could go a long way toward helping people who might otherwise suffer from diabetic retinopathy, and the device is already in clinical trials.”
Dr. Temple says she hopes that within five years her team’s transplant procedure will be in clinical trials, and their methods for activating the RPE stem cells that already exist inside the eye will be moving toward a clinical trial as well. “It’s a very exciting time to be part of this field,” she says. “I can see a future in which regenerative medicine is one of the services doctors offer their patients, and I can see doctors specializing in this type of stem-cell-based therapy. In the meantime, I think what stem cells can tell us about human cells and health and disease is enormously promising.” REVIEW
1. Krohne TU, Westenskow PD, Kurihara T, et al. Generation of retinal pigment epithelial cells from small molecules and OCT4-reprogrammed human induced pluripotent stem cells. Stem Cells Transl Med 2012;1:96–109.
2. Westenskow PD, Bucher F, Bravo S, et al. iPSC-Derived Retinal Pigment Epithelium Allografts Do Not Elicit Detrimental Effects in Rats: A Follow-Up Study. Stem Cells Int 2016;2016:8470263.
3. Zhao T, Zhang Z, Westenskow PD, et al. Humanized Mice Reveal Differential Immunogenicity of Cells Derived from Autologous Induced Pluripotent Stem Cells. Cell Stem Cell 2015;17;353–359.
4. Lin H, Ouyang H, Zhu J, et al. Lens regeneration using endogenous stem cells with gain of visual function. Nature 2016:doi:10.1038/nature17181.