|
Asheesh Tewari, MD, and |
Cataract surgery with phacoemulsification is one of the most successful surgical procedures performed, and its popularity has decreased operating times and given patients a shorter postoperative healing period. However, sight-threatening complications that involve the posterior segment can occur. The complication rate after cataract surgery is relatively low, but it is important to recognize these complications early and treat them appropriately. In this article, we will review the four major posterior segment complications of cataract surgery: retained lens fragments, postoperative endophthalmitis, pseudophakic retinal detachment, and cystoid macular edema.
Retained Lens Fragments
With a switch from traditional extracapsular cataract surgery to phacoemulsification, there has been an increase in the incidence of posterior dislocation of lens material into the vitreous cavity. Overall, the rate of retained lens fragments (RLF) is thought to be as high as 1 percent.1 Risk factors for RLF include limited pupillary dilation, traumatic cataract, and patient movement during surgery. In addition, disorders that predispose to zonular weakness, such as pseudoexfoliation and Marfan syndrome, can be associated with RLF.
The primary ocular effects of RLF are pronounced inflammation and intraocular pressure elevation. The degree of inflammatory response of the eye appears to be proportional to the size of the lens fragment.2 Typically, cortical remnants expand upon hydration in the vitreous cavity and tend to appear larger. Retained nuclear material is usually associated with a greater inflammatory response and higher intraocular pressures. The elevation of IOP is thought to occur because of liberated lens proteins and enlarged macrophages disrupting the trabecular meshwork. Over the long term, chronic inflammation can lead to development of peripheral anterior synechiae and chronic angle-closure glaucoma.
Once lens fragments have gone posteriorly during cataract surgery, the cataract surgeon should not attempt to retrieve these fragments in the vitreous; these aggressive endeavors can lead to vitreous traction at the vitreous base and put the patient at risk for retinal tears and detachments. Instead, a limited anterior vitrectomy and removal of cortical material in the anterior segment should be performed. Depending on the integrity of the posterior capsule, either an anterior chamber intraocular lens (ACIOL) or a sulcus-based posterior chamber intraocular lens (PCIOL) should be placed. Also, a silicone-based intraocular lens should be avoided because of the risk of view-limiting posterior condensation on the lens during a potential air-fluid exchange in vitrectomy surgery. The corneal incision should be sutured securely so wound leaks are avoided during subsequent surgeries. Postoperative management involves frequent topical corticosteroids, cycloplegics, and IOP-lowering medications. Prompt referral to a vitreoretinal surgeon is recommended.
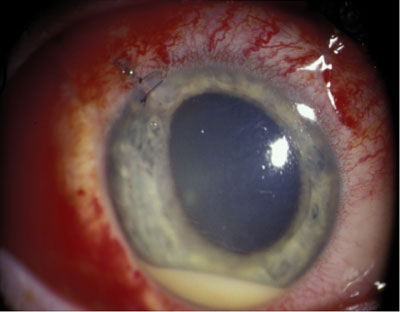 |
| Figure 1. Hypopyon in an eye with endophthalmitis. |
Treatment decisions are based upon the status of inflammation and IOP. In cases in which there is minimal cortical material, and controlled inflammation and IOP, observation can be considered. In situations when IOP is difficult to control, pars plana vitrectomy (PPV) and pars plana lensectomy (PPL) should be considered. In almost all cases of retained nuclear material, PPV/PPL should be performed. Displacement of the whole crystalline lens with the intact capsule typically does not require immediate treatment, since the inflammatory response is usually minimal, but eventually PPV/PPL will be needed to remove the entire lens.
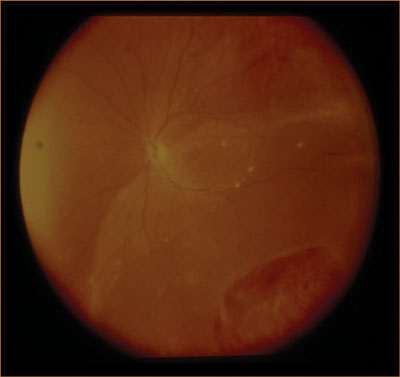 |
| Figure 2. An inferior retinal detachment. |
There is limited prospective data on observation vs. PPV in the management of RLF and also on the timing of PPV (early vs. delayed). There is little evidence in the literature that supports the idea that PPV on the same day of cataract surgery is associated with an improved outcome. In addition, there can be corneal edema from the cataract surgery, which can inhibit the view of the posterior segment, and a delay in PPV may allow the cornea to heal. The best strategy is to follow the patient clinically and intervene early within the first two weeks if inflammation and/or IOP are difficult to control.
Potential long-term complications of RLF include retinal detachment and progression of diabetic retinopathy in diabetic patients. In diabetics, the severe inflammatory response affects the blood-retinal barrier and increases permeability of the endothelial cells, predisposing to diabetic edema and proliferation. (Tewari A, et al. Invest. Ophthalmol. Vis. Sci. 2002 43: E-Abstract 3472.)
Results from a large series of patients with RLF managed by PPV demonstrated that an overall final visual acuity of 20/40 or better was achieved in 44 percent of patients.3 The study discovered the common causes of vision loss in cases of RLF were pre-existing ocular conditions, corneal edema, cystoid macular edema, and retinal detachment.
The key points in managing RLF are restraint on the part of the cataract surgeon in removing lens fragments from the vitreous cavity, aggressive treatment of inflammation and elevated intraocular pressure, and timely referral to a vitreoretinal surgeon. With proper intraoperative and postoperative management of retained lens fragments, good visual outcomes can be achieved.
Postoperative Endophthalmitis
Acute postoperative endophthalmitis is a rare but serious complication of cataract surgery. It usually presents within a week of cataract surgery and symptoms include pain and decreased vision. Findings include aqueous cells and flare that is worse than usual during the postoperative period, and fibrinous reaction with hypopyon (See Figure 1) may also be present. The majority of cases are caused by coagulase-negative, gram-positive species such as Staphylococcus epidermidis. Other species such as Staphylococcus aureus, Streptococci, and gram-negative organisms account for approximately 25 percent of cases of postoperative endophthalmitis. The incidence is estimated to be 0.1 percent but is thought to be rising.4 A recent report found a statistically increased risk with clear corneal incisions (0.29 percent) compared with scleral tunnel incisions (0.05 percent).5
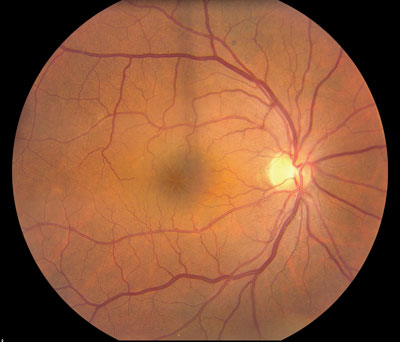 |
| Figure 3. Color photograph demonstrating fluid-filled spaces in cystoid macular edema. |
The most common source of postoperative endophthalmitis is the patient's eyelids and ocular surface flora. Although there is limited data supporting efficacy of topical antibiotic prophylaxis for the prevention of postoperative endophthalmitis, its use is still present. Fluoroquinolones tend to have broad coverage, a favorable safety profile, and adequate penetration into the eye and, thus, are preferred for treatment. However, the only strategy that demonstrates reduction in rates of postoperative endophthalmitis is external preparation and ocular surface irrigation with topical 5% povidone iodine.6
Once endophthalmitis is diagnosed, the Endophthalmitis Vitrectomy Study (EVS) guides management.7 Treatment involves injection of intravitreal antibiotics with either a vitreous biopsy ("tap") or PPV. Intravitreal injections pass the blood-retinal barrier and can achieve rapid therapeutic drug levels at the sites of infection. Intravitreal injections of vancomycin (1 mg/0.1cc) and ceftazidime (2.25 mg/0.1cc) are well-tolerated and give good coverage of the organisms typically involved in postoperative endophthalmitis. The use of concomitant steroids to reduce the inflammatory component is controversial.
Initial visual acuity is considered the best predictor of final visual outcome. In fact, the level of vision on presentation aids in determining the treatment. In patients with hand-motion vision or better, treatment usually involves a vitreous tap and injection of intravitreal antibiotics. The vitreous fluid that is removed is sent for gram stain and culture. In some cases, the vitreous is dense and intermixed with inflammatory membranes, and an adequate vitreous sample cannot be obtained safely ("dry tap"). In this case, an aqueous sample can be obtained via an anterior chamber paracentesis. Cultures from the vitreous tap are positive in 56 to 70 percent of the cases, compared with only 40 percent from the paracentesis.8 In patients with light-perception vision, a PPV with injection of intravitreal antibiotics is recommended. Vitrectomy allows removal of the infecting organisms, excision of vitreous membranes that could lead to subsequent tractional detachment of the retina and improved penetration ability of antibiotics.9 Samples of vitreous are taken at the time of PPV and sent for culture studies. Also, patients who show no clinical improvement after vitreous tap and injection should have a PPV done followed by repeat intravitreal antibiotic injection.
The EVS demonstrated that systemic postoperative antibiotic therapy has no benefit in the treatment of endophthalmitis.7 However, the antibiotics used in the study (ceftazidime and amikacin), have limited coverage for gram-positive organisms. Newer antibiotics, such as the fourth-generation fluoroquinolones, have greater penetration when given systemically and have broader coverage. One of these agents, moxifloxacin, has been studied with different delivery techniques. Moxifloxacin has been found to have oral bioavailability of greater than 90 percent with achievement of peak plasma concentrations in one to two hours after oral administration. Orally administered moxifloxacin achieves therapeutic aqueous and vitreous levels in the non-inflamed human eye.10 Topically administered moxifloxacin achieves higher aqueous concentrations than vitreous concentrations, but the levels achieved may be beneficial for prophylaxis.11 Moxifloxacin can also be delivered to the eye via a dissolvable corneal collagen shield and high aqueous levels can be achieved in the first few hours after surgery.12 The activity spectrum of moxifloxacin encompasses the most frequently encountered causative bacterial species in endophthalmitis. However, to accurately evaluate the benefit of newer systemic and topical medications for postoperative endophthalmitis, a large randomized trial would be necessary.
Pseudophakic Retinal Detachment
Pseudophakic patients have a 5.5 times higher risk of retinal detachment than phakic patients, and an overall incidence of 1 percent per year.13,14 Posterior vitreous detachment (PVD) is more common in patients who have undergone cataract surgery. The exact mechanism of this is not known but anterior vitreous may be disturbed during uncomplicated cataract surgery, leading to structural changes in the remainder of the vitreous. Also, it may be that protuberance of the posterior surface of the lens is an important factor in reducing vitreous traction on the peripheral retina during ocular movements.
Risk factors for retinal detachment in pseudophakic patients include vitreous loss during cataract surgery, high myopia, lattice degeneration, and history of prior retinal detachment in the fellow eye. The majority of pseudophakic retinal detachments occur during the first year after cataract surgery. Patients may present with symptoms of floaters, flashes of light, and visual field defects. Fundus examination typically reveals small anteriorly located horseshoe tears with an associated retinal detachment. These pseudophakic detachments tend to progress rapidly and are more likely to involve the macula.
Multiple surgical options exist for the repair of a pseudophakic retinal detachment, including pneumatic retinopexy, scleral buckle, pars plana vitrectomy, and combined pars plana vitrectomy and scleral buckle. Surgeon preference varies but generally for pseudophakic patients with a retinal detachment, a pars plana vitrectomy is the surgical procedure of choice. The absence of a crystalline lens avoids the issues of cataract progression after vitrectomy and more importantly, a more thorough peripheral vitrectomy can be performed, without risk of damaging a crystalline lens. For inferior detachments in pseudophakic individuals (See Figure 2), combined pars plana vitrectomy and scleral buckle may be used, since the encircling band can be used to provide additional support to the vitreous base inferiorly.
Cystoid Macular Edema
Cystoid macular edema is a common cause of visual decline after uncomplicated cataract surgery. CME typically presents four to six weeks after cataract surgery, and its course can fluctuate. Clinical CME is described as vessel leakage with visual acuity of 20/40 or worse. Sub-clinical angiographic CME may not be associated with significant visual loss, but fluorescein angiography and optical coherence tomography may detect macular edema. Angiographic CME occurs in 20 percent of pseudophakic eyes, and the risk of CME is higher in eyes that have had vitreous loss. However, CME is visually significant in 7 to 12 percent of pseudophakic patients.16 Although most cases of CME resolve spontaneously within six to 12 weeks, if the edema persists for greater than six months, it is referred to as chronic CME.
The mechanism of CME development is thought to be inflammatory and/ or mechanical in nature. One theory involves the prostaglandin-mediated breach of the blood-retinal barrier, leading to increased permeability of retinal capillaries and subsequent leakage. Prostaglandins can be released by the surgery itself and also by interaction between parts of the intraocular lens and the iris. Mechanically, the vitreous can exert traction on the macula and on the retinal vessels, causing leakage. Clinical examination of the macula often reveals fluid-filled spaces surrounded by smaller cysts or a yellowish spot in the central fovea (See Figure 3). In addition, there may be vitreous incarceration to the cataract wound or abnormal positioning of the IOL. Fluorescein angiography reveals leakage of perifoveal capillaries and late-frame images demonstrate a classic petaloid pattern and associated hyperfluorescence of the optic nerve (See Figure 4). A few risk factors for CME include pre-existing ocular inflammation, diabetic retinopathy, presence of epiretinal membrane or vitreoretinal traction, vitreous loss, retained lens fragments, and a history of CME in the fellow eye.
Current treatment strategies are focused on inhibiting components in the prostaglandin pathway. Topical non-steroidal anti-inflammatory agents, such as ketorolac, inhibit the enzyme cyclooxygenase, which in involved in prostaglandin production. Steroids primarily act on phospholipase A2, thus inhibiting the release of arachidonic acid. Concurrent administration of steroids and NSAIDs has been shown to provide synergistic activity that can result in resolution of CME.16 For the active treatment of CME, topical steroids and NSAIDs should be used for one to three months and tapered slowly as CME resolves. Vitreous incarceration in the wound may be treated with Nd:YAG laser vitreolysis, and in cases of vitreomacular traction (VMT), pars plana vitrectomy may be needed.
Topical ketorolac administered four times daily has been shown to be effective in preventing post-surgical angiographic CME. For patients with risk factors for CME development, topical NSAID therapy should be begun one week preoperatively and continued for four to eight weeks postoperatively. For patients not at-risk, a topical NSAID can be given one to two days preoperatively and continued postoperatively for four weeks. Topical nepafenac is a new NSAID that is being advocated for CME. It is a prodrug that is metabolically converted to a cyclooxygenase inhibitor, amfenac, by intraocular hydrolases. This form of the medication is thought to have improved penetration to the posterior segment over conventional NSAIDs, but there is no significant clinical data that has demonstrated this yet.
Intravitreal triamcinolone acetonide has been used increasingly for cases of recalcitrant CME. Delivering steroid directly into the eye permits excellent penetration to the retina, and the 4-mg dose can achieve a 50 percent decrease in central macular thickness.18 However, the effects can be short term and the associated risks are cataract progression, IOP elevation, retinal tear and endophthalmitis.18 Alternatively, triamcinolone acetonide can be given by injection into the sub-Tenon's space, in addition to continuing topical NSAID therapy. Although achieving adequate penetration can be an issue, the risks of intravitreal injection are minimized by this method.
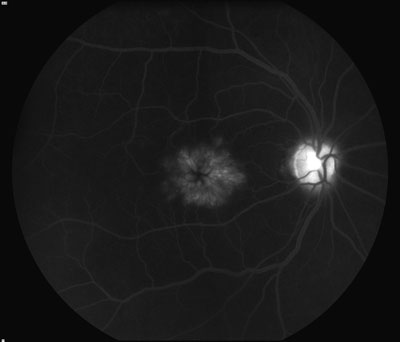 |
| Figure 4. Fluorescein angiogram showing the classic petaloid appearance of cystoid macular edema. |
Thankfully, with technological advances in phacoemulsification and improved perioperative delivery of care, the rate of posterior segment complications in cataract surgery is low. However, when these complications do occur, prompt recognition and referral for treatment allows us to provide the best care for our patients.
Dr. Tewari is a vitreoretinal fellow at the Barnes Retina Institute, Washington University Department of Ophthalmology and Visual Science. Dr. Shah is from the Barnes Retina Institute and is a clinical associate professor with Washington University Department of Ophthalmology and Visual Science. Contact Dr. Shah at Barnes Retina Institute, 1600 S. Brentwood Blvd, Suite 800, St. Louis, Missouri, 63144. Phone: (314) 367-1278, fax: (314) 962-2770, E-mail: gkshah1@gmail.com.
1. Pande M, Dabbs TR. Incidence of lens matter dislocation during phacoemulsification. J Cataract Refract Surg 1996;22:737-42.
2. Gilliland GD, Hutton WL, Fuller DG. Retained intravitreal lens fragments after cataract surgery. Ophthalmology 1992;99:1263-7.
3. Scott IU, Flynn HW Jr., Smiddy WE, et al. Clinical features and outcomes of pars plana vitrectomy in patients with retained lens fragments. Ophthalmology 2003;110:1567-72.
4. Eifrig CW, Flynn HW Jr., Scott IU, Newton J. Acute-onset postoperative endophthalmitis: Review of incidence and visual outcomes (1995-2001). Ophthalmic Surg Lasers 2002;33:373-8.
5. Nagaki Y, Hayasaka S, Kadoi C, et al. Bacterial endophthalmitis after small-incision cataract surgery. effect of incision placement and intraocular lens type. J Cataract Refract Surg 2003;29:20-6.
6. Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology 2002;109:13-24.
7. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol 1995;113:1479-96.
8. Kim JE, Flynn HW, Jr., Rubsamen PE, et al. Endophthalmitis in patients with retained lens fragments after phacoemulsification. Ophthalmology 1996;103:575-8.
9. Sunaric-Megevand G, Pournaras CJ. Current approach to postoperative endophthalmitis. Br J Ophthalmol 1997;81:1006-15.
10. Hariprasad SM, Shah GK, Mieler WF, et al. Vitreous and aqueous penetration of orally administered moxifloxacin in humans. Arch Ophthalmol 2006;124:178-82.
11. Hariprasad SM, Blinder KJ, Shah GK, et al. Penetration pharmacokinetics of topically administered 0.5% moxifloxacin ophthalmic solution in human aqueous and vitreous. Arch Ophthalmol 2005;123:39-44.
12. Hariprasad SM, Shah GK, Chi J, Prince RA. Determination of aqueous and vitreous concentration of moxifloxacin 0.5% after delivery via a dissolvable corneal collagen shield device. J Cataract Refract Surg 2005;31:2142-6.
13. Rowe JA, Erie JC, Baratz KH, et al. Retinal detachment in Olmsted County, Minnesota, 1976 through 1995. Ophthalmology 1999;106:154-9.
14. Powe NR, Schein OD, Gieser SC, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Cataract Patient Outcome Research Team. Arch Ophthalmol 1994;112:239-52.
15. Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc 1998;96:557-634.
16. Heier JS, Topping TM, Baumann W, et al. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology 2000;107: 2034-8.
17. Benhamou N, Massin P, Haouchine B, et al. Intravitreal triamcinolone for refractory pseudophakic macular edema. Am J Ophthalmol 2003;135: 246-9.
18. Jager RD, Aiello LP, Patel SC, Cunningham ET Jr. Risks of intravitreous injection: A comprehensive review. Retina 2004;24:676-98.




