Toxoplasmosis is an infection caused by the parasite Toxoplasma gondii, which exists in three major forms: the oocyst, the tachyzoite or infectious form, and the tissue cyst or latent form. T. gondii has a complex life cycle. Cats are the definitive hosts and typically become infected by consuming tissue cysts present within the flesh of an infected intermediate hosts, such as small mammals or birds. The parasite reproduces sexually within the cat's intestine and oocysts are then excreted in the cat's feces to contaminate the cat's litter box and surrounding environment. Oocysts can persist for more than a year under the right conditions.
Humans usually contract toxoplasmosis in one of three principal ways. First, transmission can be caused by ingestion of undercooked, infected meat containing T. gondii tissue cysts. Second, toxoplasmosis can be acquired by inadvertent contact with cat feces, cat litter or soil containing oocysts. And third, transplacental transmissions may occur when a pregnant woman contracts a primary infection. In transplacental transmission the tachyzoites cross the placenta and then travel via blood and lymph. The tachyzoites then encyst in the infected tissues. Congenital disease tends to be more severe when acquired in the first trimester of pregnancy, but transmission rates are highest in the third trimester. Congenital disease may manifest in a variety of ways from spontaneous abortion, stillbirth and severe multisystem involvement to asymptomatic infants who only present later in life with ocular and neurological scars and/or reactivation. Chronic or recurrent maternal infection is generally not thought to confer risk of congenital toxoplasmosis. Isolated reports of epidemic outbreaks following inhalation of aerosolized cysts or ingestion of contaminated drinking water suggest that there may be additional means of contracting toxoplasmosis in highly endemic areas.1
Ocular Toxoplasmosis
While it was previously thought that congenital toxoplasmosis accounted for the majority of cases of toxoplasmic retinochoroiditis seen in clinical practice, it has now been established that acquired infection is the most important and more common source of ocular infection.1,2
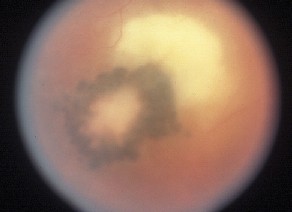
The symptoms associated with ocular toxoplasmosis are usually unilateral and include hazy or blurred vision, frequently accompanied by floaters. An iritis is typically present on slit-lamp examination and can be granulomatous in appearance. Ten to 20 percent of patients will have acutely elevated intraocular pressure.2,3 Classically, there is a moderately dense vitritis associated with a white active chorioretinal lesion ("headlight in the fog") and an adjacent or nearby chorioretinal scar (See Figure 1). Chorioretinal scarring is not always present, however, in which case an acquired infection should be suspected. Additional ocular complications can include cataract, optic disc edema, CME, retinal vasculitis, serous retinal detachment, and choroidal neovascularization.2,4,5 The course of disease depends on the immune status of the infected individual and route of infection.1,2,6 Toxoplasmosis acquired after birth is typically unilateral and runs a self-limiting course. If present, systemic symptoms tend to be mild and non-specific. The ocular lesion is frequently asymptomatic, with disease first becoming apparent during recurrent inflammation in later life.3,4 Immunocompromised and older patients are at risk for large, multiple and/or bilateral lesions.6 In addition, in immunosuppressed patients, the ocular inflammation is often associated with a symptomatic systemic illness.6 Signs and symptoms can include fever, malaise, maculopapular rash, lymphadenitis, dyspnea, myositis, acute myocarditis and encephalitis.1,2,6
|
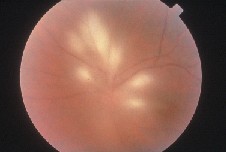
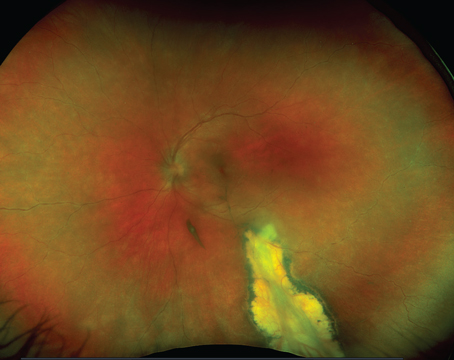
Ocular toxoplasmosis in immunosuppressed individuals may be unilateral or bilateral with single or multiple chorioretinal lesions (See Figure 2). Other atypical presentations that may occur include punctate outer retinal toxoplasmosis, retinal vasculitis, retinal vascular occlusions, rhegmatogenous and serous retinal detachments, pigmentary retinopathy, papillitis, neuroretinitis and scleritis.6
|
While in many instances indirect ophthalmoscopic examination alone is all that is needed to make the diagnosis of toxoplasmic retinochoroiditis, the recognition of atypical forms of the disease can be challenging and often requires the use of serological testing. Most laboratories use an enzyme-linked immunosorbent assay (ELISA) or immunofluorescent antibody (IFA) test to measure serum immunoglobulin M (IgM) and immunoglobulin G (IgG) antibody titers directed against T. gondii. IgG antibodies are produced within the first couple of weeks post-infection and typically remain positive for life.4,5,7 IgM antibodies, in contrast, are known to rise early during the acute stage of the infection and may be useful in diagnosing acute acquired toxoplasmosis. IgM antibodies typically remain positive for less than one year.7
Having a positive IgG signifies only that the patient has at some time in the past been exposed to T. gondii, but does not differentiate latent versus active infection.2,4,7 Moreover, interpretation of these tests can be confusing, since a high proportion of the world's population has been exposed to, and is therefore seropositive for, T. gondii. IgM does not cross the maternal-placental blood barrier, so this immunoglobulin may be useful in differentiating congenital versus acquired disease. A negative anti-T. gondii IgG serum antibody titer can be taken as fairly strong evidence against toxoplasmosis as the cause of retinitis.
Direct isolation of T. gondii tachyzoites in body fluid, or tachyzoites or bradyzoites from tissue, allows for definitive diagnosis, but is usually not available when considering the diagnosis of ocular toxoplasmosis. Polymerase chain reaction (PCR) has been used successfully to examine aqueous and vitreous fluid for toxoplasmic DNA6,7,8 and may be particularly useful in patients with an atypical presentation.6,8
Treatment
Treatment of ocular toxoplasmosis depends largely on the host's immune status and the clinician's assessment of the location and severity of the infection and accompanying inflammation. In immunocompetent patients, the disease tends to be self-limiting. Numerous criteria have been suggested, however, to help determine when and how to initiate therapy. Factors to consider include vision, the location of the lesion relative to the macula and optic disc, the number and size of lesions, and the severity and duration of the vitreous inflammation.2,5
Numerous agents have been used to treat toxoplasmosis over the years, and there is no single regimen that can be applied to every patient. In a recent survey of the American Uveitis Society (AUS), 78 uveitis specialists used 24 different regimens, utilizing nine parasitic drugs.2 The most common drugs utilized in typical cases of infection for an average adult were pyrimethamine (loading dose: 50-100 mg; treatment dose: 25-50 mg daily), sulfadiazine (loading dose: 2-4 g; treatment dose: 1.0 g four times daily), Clindamycin (treatment dose: 300 mg four times daily), trimethoprim/sulfamethoxazole (treatment dose: 160 mg/800 mg twice daily) and prednisone (treatment dose: 20-80 mg daily, tailored to the severity of the inflammation). Combinations of the above drugs were commonly employed. The regimen used most often was pyrimethamine, sulfadiazine and prednisone (triple therapy). Some clinicians advocated adding Clindamycin to this regimen (quadruple therapy).2
Sulfonamides and pyrimethamine work by inhibiting folic acid metabolism. It is important, therefore, that folinic acid (5 mg every other day) be added to any treatment regime incorporating these two drugs. In the same survey of the 78 AUS physicians, only 17 percent of the respondents used an oral corticosteroid when treating all cases of ocular toxoplasmosis in immunocompetent patients.2 Other physicians used corticosteroid only for specific indications, including severe vitritis, decreased visual acuity, and close proximity of the lesion to the optic nerve and/or fovea. Thirty-six percent of the respondents started antiparasitic therapy simultaneously with corticosteroids and 64 percent of the physicians waited up to seven days to start corticosteroid therapy.2 The use of long-acting periocular and intraocular corticosteroid formulations, such as triamcinolone acetonide (Kenalog), should be avoided, as these have been associated with prolonged activation, panophthalmitis, and even loss of the eye.9
When treating pregnant women for active ocular toxoplasmosis, most physicians avoid the use of pyrimethamine, given its possible teratogenic effects. In contrast, spiramycin (treatment dose: 400 mg t.i.d.) can be used in pregnant woman and may reduce the rate of tachyzoite transmission to the fetus.2
Brazilian researcher Claudio Silveira, MD, and coworkers recently described the use of long-term intermittent trimethoprim/sulfamethoxazole to decrease the risk of reactivation in patients with recurrent toxoplasmic retinochoroiditis.10 Unlike other sulfa formulations, trimethoprim/sulfamethoxazole is readily available at most pharmacies. Trimethoprim/sulfamethoxazole is also inexpensive and associated with a relatively low drug allergy rate, with slightly more than 5 percent of the treated patients in this study having developed cutaneous erythema, which resolved when drug treatment was discontinued.
Lotje Bosch-Driessen, MD, PhD, and colleagues described an association between cataract surgery and an increased risk of reactivation of otherwise inactive toxoplasmic retinochoroiditis.11 This has prompted some ophthalmologists to consider prophylactic antimicrobial treatment shortly before and after surgery in those patients with inactive toxoplasmic scars, particularly those that threaten the optic disc or fovea. While there is currently no consensus regarding which regimen to use in such patients, trimethoprim/sulfamethoxazole for three days prior and two to three weeks after surgery would seem to be a reasonable choice unless otherwise contraindicated.
Drs. Wertheim and Smith practice at the Casey Eye Institute, Oregon Health & Science University, 3375 SW Terwilliger Blvd., Portland, Ore. 97239. Contact Dr. Smith at (503) 494 5203, or
smithjus @ohsu.edu. Dr. Cunningham is a professor of ophthalmology and director of the Uveitis Service at New York University School of Medicine. He is also senior vice president, Medical Strategy, at Eyetech Pharmaceuticals. Dr. Cunningham practices at Vitreous-Retina-Macula Consultants of New York, 460 Park Ave. 5th floor, New York, N.Y. 10021. Contact him at (212) 824 3130, or
emmett_cunningham@yahoo.com.
1. Holland GN. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol. 2003;136:973-88.
2. Holland GN, Lewis KG. An update on current practices in the management of ocular toxoplasmosis. Am J Ophthalmol 2002;134:103-114
3. Hovakimyan A, Cunningham ET Jr. Ocular toxoplasmosis. Ophthalmol Clin N Am 2002;15:327-332
4. Rothova A. Ocular manifestations of toxoplasmosis. Curr Opin Ophthalmol. 2003 Dec;14:384-8.
5. Bosch-Driessen LE, Berendschot TT, Ongkowuwito JV, Rothova. Ocular toxoplasmosis: Clinical features and prognosis of 154 patients. Ophthalmology 2002; 109(5):869-878.
6. Smith JR, Cunningham ET Jr. Atypical presentations of ocular toxoplasmosis. Curr Opin Ophthalmol 2002;13:387-392
7. Holland GN. Ocular Toxoplasmosis: new directions for clinical investigations. Occ Immun Inflam 2000;8:1-7.
8. Montoya JG, Parmley S, Liesenfeld O, et al. Use of polymerase chain reaction for diagnosis of ocular toxoplasmosis. Ophthalmology 1999;106:1554-1563
9. Bosch-Driessen LH, Rothova A. Sense and nonsense of corticosteroid administration in the treatment of ocular toxoplasmosis. Br J Ophthalmol. 1998 Aug;82:858-60.
10. Silveira C, Belfort R Jr, Muccioli C, Holland GN, et al. The effect of long-term intermittent trimethoprim/sulfamethoxazole treatment on recurrences of toxoplasmic retinochoroiditis. Am J Ophthalmol 2002; 134:41-46.
11. Bosch-Driessen LH, Plaisier MB, Stilma JS, et al. Reactivations of ocular toxoplasmosis after cataract extraction. Ophthalmology 2002:109:41-44.