“All of these devices have a small footprint on the eye, are very focused on the tissue being treated, and don’t cause significant collateral damage. Many of them are also coupled with cataract surgery,” says Malik Kahook, MD, who is a professor of ophthalmology at the University of Colorado School of Medicine.
According to Leonard Seibold, MD, when compared to filtering surgeries, MIGS may not be quite as effective at achieving very low IOP goals. “However, they are very effective for treating mild to moderate glaucoma that doesn’t require such low target pressures in a much safer manner. Additionally, the surgery itself is more efficient, there is less risk for complications, and vision recovery is faster,” adds Dr. Seibold, an assistant professor of ophthalmology at the University of Colorado School of Medicine.
Because of their safety, MIGS are typically used earlier in the disease process than other glaucoma procedures. “Our traditional glaucoma surgeries have been fraught with the potential for multiple, vision-threatening complications. Historically, we have waited until end-stage disease in many cases to do glaucoma surgery because the risk/benefit analysis was skewed due to the possibility of vision-threatening complications. Now, with MIGS, we can intervene surgically a lot sooner in a safer manner, and patients can be less reliant on medications, with better quality of life,” Dr. Seibold explains.
iStent
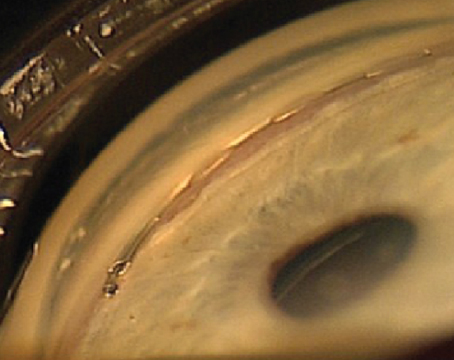
 |
| Figure 1. iStent implanted in the eye. Leonard Seibold, MD |
The iStent trabecular micro-bypass implant (Glaukos) is the only FDA-approved device for the treatment of mild to moderate open-angle glaucoma. It is the first MIGS implant to improve the eye’s natural fluid outflow by creating a permanent opening in the trabecular meshwork to lower IOP. It can be safely implanted in the eye during cataract surgery, and it spares important eye tissue that is often damaged by traditional surgeries. Interestingly, it is the smallest medical device ever approved by the FDA, and it can be implanted through a 1.5-mm corneal incision.
A recent German study found that implantation of the iStent during cataract surgery was safe and effective as measured by sustained IOP reduction and reduced medication use.1 Additionally, it demonstrated an excellent safety profile through three years postoperatively. In this prospective, open-label, non-randomized study, a single iStent was implanted through the same temporal, limbal incisions used for cataract surgery in 62 eyes of 43 consecutive patients. A total of 41 eyes have been followed for three years postoperatively. Mean preoperative IOP was 24.1 ±6.9 mmHg on a mean of 1.8 ±0.9 medications. Eyes with no secondary surgical intervention demonstrated a mean IOP reduction to 14.8 ±4.2 mmHg at 12 months postoperatively, 14.5 ±2.2 mmHg at 24 months, and 14.9 ±2.3 mmHg at 36 months. At 36 months postoperatively, medications were eliminated in 74 percent of eyes. There were no intraoperative or postoperative complications.
Xen45
The Xen stent (Allergan) is made of a soft, collagen-derived gelatin. It is 6 mm long and is approximately the width of a human hair. The stent is injected through a small self-sealing corneal incision using a simple, preloaded IOL-like injector. After being implanted in the eye, it creates a gentle outflow of aqueous from the anterior chamber into the subconjunctival space. It has CE Mark internationally, but is not FDA-approved for use in the United States.
A study conducted in Spain found that cataract surgery combined with Xen45 implant surgery effectively reduces IOP and the number of IOP-lowering medications in eyes with mild to moderate open-angle glaucoma.2 This prospective study included 30 eyes that required cataract surgery and at least two medications to control IOP. Surgery was performed through two temporal incisions, separated by 90°, using the inferior to implant the Xen45 into the superior nasal region. Best-corrected visual acuity was 0.37 ±0.2 before surgery and 0.72 ±0.15 12 months after surgery. Additionally, preoperative IOP was 21.2 ±3.4 mmHg with 3.07 medications. IOP decreased by 61.65 percent at one day, 37.26 percent at one month, 35.05 percent at three months, 31 percent at six months, 30.6 percent at nine months, and 29.34 percent at 12 months. And, the number of medications decreased by 94.57 percent.
“This device was originally developed by AqueSys, and the company was just picked up by Allergan,” Dr. Kahook says. “I believe it is the closest to FDA approval. This is a full-thickness ab interno device, and it may or may not be coupled with cataract surgery. Basically, this device is introduced with a small needle across the anterior chamber, through the sclera, into the sub-Tenon space. When the needle is retracted, the device is left behind so that it connects the sub-Tenon space to the anterior chamber. Available data show that it might lower pressure more than the iStent and some of the other MIGS devices, but the safety profile is currently not clear.”
E. Randy Craven, MD, who has a joint appointment at Johns Hopkins and at the King Khaled Eye Specialist Hospital in Riyadh, agrees. “This device produces a Xen bleb and a pressure reduction similar to what can be achieved with trabeculectomies. But, it has a different bleb appearance than a trabeculectomy bleb,” he says.
CyPass Micro-Stent
The CyPass Micro-Stent was developed by Transcend Medical, which was purchased in February by Alcon Laboratories. This micro-stent is a supraciliary device designed to create a controlled outflow pathway to the suprachoroidal space. The device is a 6.35-mm-long tube made of a polyimide material with an outer diameter of 0.51 mm. It can be placed through a 1.5-mm corneal incision and is inserted on a small guidewire with a special tip that separates the iris from the scleral spur. The CyPass Micro-Stent is inserted into the cleft that’s created, and the openings along the length of the tube allow aqueous to flow out. This device has CE Mark approval, but is not yet FDA-approved in the United States. Dr. Kahook believes that it could potentially be approved sometime in the next year or two.
“This is a suprachoroidal device, which is a separate category of MIGS devices, and it should be the first to market for this unique category,” Dr. Kahook says. “It is being explored as a tandem device with cataract surgery, which would be performed either before or after implantation of this device. It is implanted ab interno under gonioscopic view. The device goes in across the anterior chamber and is then inserted in the suprachoroidal space. I have not implanted this particular device, but I have implanted suprachoroidal devices in the past and found them to be relatively straightforward to position.”
 |
| Figure 3. The Kahook dual blade removing trabecular meshwork under gonioscopic view. Malik Kahook, MD |
“All of the data that have been published so far show that it results in a 30 percent reduction in IOP. It seems to have a really good safety profile, and it looks very encouraging. I am personally encouraged about it because I went to Africa to the Sinskey Eye Institute, and we taught some of the African surgeons how to implant the device. They had no trouble adopting it. It looks promising for the global treatment of glaucoma. It offers a route of pressure reduction that doesn’t rely on the outflow system of the trabecular meshwork, Schlemm’s canal, or the subconjunctival space,” Dr. Craven explains.
 |
| Figure 2. The Kahook dual blade. Malik Kahook, MD |
iStent Inject
This is the newest iteration of the iStent. It is similar in some ways to the current iStent, but the insertion is different. “It is made to be inserted by essentially projecting it directly into Schlemm’s canal in a procedure that should be easier to perform than the current iStent that’s on the market. Data are just now coming out on this device, and it is showing similar efficacy to the current iStent,” Dr. Kahook says.
A recent study has found that the iStent Inject is at least as effective as two medications at lowering IOP in open-angle glaucoma patients, and it has a highly favorable safety profile.4 The study was conducted to compare the outcomes of patients with open-angle glaucoma that was not controlled on one medication who either underwent implantation of two iStent Inject trabecular micro-bypass devices or received a fixed combination of latanoprost/timolol. Of the 192 patients enrolled in the study, 94 were randomized to surgery, and 98 were randomized to receive medical therapy. At 12 months postoperatively, 89 of the 94 eyes that underwent surgery (94.7 percent) reported an unmedicated IOP reduction of 20 percent or more, and 88 of the 98 eyes that received medical therapy (91.8 percent) reported an IOP reduction of 20 percent or more compared with baseline unmedicated IOP. Additionally, a 17.5 percent between-group difference in favor of the iStent Inject was statistically significant at lowering IOP 50 percent or more. An IOP of 18 mmHg or less was reported in 92.6 percent of eyes in the iStent Inject group and in 89.8 percent of eyes in the medical therapy group. Mean IOP decreases of 8.1 mmHg in the iStent group and 7.3 mmHg in the medical therapy group were reported, and a high safety profile was observed in both groups.
“I believe that they have closed out all of their data collection, and the FDA is considering a more rapid review, so it is probably not too far away from being approved,” Dr. Craven says. ‘The two stents over one standard iStent seem to hold some promise and hope for better pressure reduction.”
Additionally, Glaukos has developed a suprachoroidal device called iStent Supra, which is similar to the CyPass. “We don’t have a lot of public clinical information on that device, but the thought process is that it would be similar to what we are seeing with the CyPass implant,” Dr. Kahook says. “This is currently going through the FDA approval process, but is still likely years away from the U.S. market.”
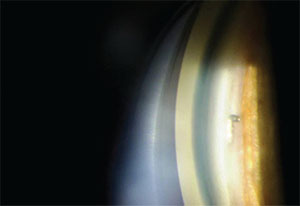
 |
| Figure 4. Alcon’s CyPass Micro-Stent. |
Hydrus
The Hydrus device (Ivantis) is an intracanalicular scaffold that is about the size of an eyelash. It is made from nitinol, a highly elastic, biocompatible alloy used in many implantable medical devices. The device is placed inside Schlemm’s canal during cataract surgery, and it increases outflow by allowing aqueous to bypass the trabecular meshwork.
A recent study assessed the safety and efficacy of the Hydrus Microstent implantation combined with cataract surgery for reducing IOP in patients with open-angle glaucoma.5 The study included 100 eyes with open-angle glaucoma in 100 patients with IOPs of 24 mmHg or less with four or fewer hypotensive medications and a washed-out diurnal IOP of 21 to 36 mmHg. Patients were randomized to receive either cataract surgery with the micro-stent or cataract surgery alone. At 24 months postoperatively, the proportion of patients with a 20-percent reduction in washed-out diurnal IOP was significantly higher in the Hydrus patients than in the patients who only underwent cataract surgery (80 percent compared with 46 percent), and the mean diurnal IOP was significantly lower in the Hydrus group than in the group that underwent cataract surgery alone (16.9 ±3.3 mmHg compared with 19.2 ±4.7 mmHg). Additionally, the number of patients using no hypotensive medications was significantly higher in the Hydrus group at 24 months (73 percent compared with 38 percent in the cataract surgery alone group).
According to Dr. Craven, the company is still gathering two-year data and is closing out the FDA trial. “All of the data I have seen have been very favorable, but approval is still probably over a year away,” he adds.
Dr. Kahook notes, “I believe the Hydrus holds great promise to lower IOP significantly by reaching three clock hours of collector channels with a single implant. I also found this device to be extremely comfortable to implant in several patients I operated on recently.”
When to Use a MIGS Device?
According to Dr. Kahook, patients are still undergoing trabeculectomy or glaucoma drainage device implantation if they have advanced glaucoma or require significant IOP lowering. MIGS devices play a more significant role when modest IOP lowering is sufficient and particularly where the main goal is to decrease dependence on topical medications. “I also believe there is a role for MIGS devices in more advanced disease if the patient and treating physician are very motivated to avoid filtration surgery,” says Dr. Kahook. “I have been surprised at times by how much IOP lowering can be achieved in a small subset of patients with more advanced disease after MIGS procedures.” He is increasingly relying on the Kahook Dual Blade (New World Medical, Rancho Cucamonga, Calif.), which he developed. “This has become our go-to surgery for lowering IOP in both pseudophakic and phakic patients. I have been impressed with the results we and others have been getting since the device was introduced in November of last year,” he adds. REVIEW
Dr. Kahook has a financial interest in as the inventor of the Kahook Dual Blade. He is an unpaid consultant to Ivantis. Dr. Kahook has received intellectual property payments from Glaukos.
Dr. Craven is a consultant to Allergan, Alcon, Ivantis and Transcend.
Dr. Seibold has served as a consultant for New World Medical and Allergan and has received research support from Alcon and Glaukos.
1. Neuhann TH. Trabecular micro-bypass stent implantation during small-incision cataract surgery for open-angle glaucoma or ocular hypertension: Long-term results. J Cataract Refract Surg 2015;41:2664-2671.
2. Perez-Torregrosa VT, Olate-Perez A, Cerda-Ibanez M, et al. Combined phacoemulsification and XEN45 surgery from a temporal approach and 2 incisions. Arch Soc Esp Oftalmol 2016; E-pub ahead of print.
3. Hoeh H, Vold SD, Ahmed IK, et al. Initial clinical experience with the CyPass Micro-Stent: Safety and surgical outcomes of a novel supraciliary microstent. J Glaucoma 2016;25:106-112.
4. Fea AM, Belda JI, Rekas M, et al. Prospective unmasked randomized evaluation of the iStent inject versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol 2014;8:875-882.
5. Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology 2015;122:1283-1293.