Potential Diseases
Currently, gene therapy operates by injecting the eye with a virus, called a vector, that carries either a healthy gene that will replace the gene that’s missing in a certain eye disease, or which enters retinal cells and turns them into “biofactories” that produce a protein that helps treat a disease. Though these approaches are being tried in different ailments, experts say that there are certain diseases that are likely candidates to have the first approved gene therapies in the United States in the next few years.
“I think the one that’s most likely to be approved is gene augmentation therapy for the RPE65 form of Leber congenital amaurosis, or for other conditions caused by RPE65 deficiency,” says Jean Bennett, MD, PhD, the F.M. Kirby professor of ophthalmology at the Perelman School of Medicine at the University of Pennsylvania, and one of the first researchers to use viral vector-based gene therapy to reverse blindness. She currently is the scientific director of the Phase III human trial of gene therapy for LCA. “I think others will follow, though it’s hard to predict the one that will be next because they’re all still in Phase I trials. However, I am excited about the data for the treatment of choroidal neovascular complications of age-related macular degeneration using sFlt-1, the receptor for VEGF. And I’m very interested in the studies that are ongoing for Leber hereditary optic neuropathy, and optogenetic therapy. Perhaps therapy for choroideremia would be the second one approved, since that research seems to be moving quickly now.” Spark Therapeutics (Philadelphia) is sponsoring the LCA and the choroideremia trials working with the RPE65 gene.
Robert MacLaren, FRCOphth, FRCS, professor of ophthalmology at the University of Oxford in the United Kingdom, says certain types of retinal disease are more amenable to current gene therapy approaches than others, and that might hold true for the next five years. “First, the diseases that are due to deficiencies of genes are probably easier to treat than those in which the genes are causing detrimental effects,” he says. “This is because we know a little bit more about gene replacement than gene knockout. Second, a gene that has such a severe effect on the eye that it affects the development of the retina and the normal structure of the eye at birth, such as in Norrie disease, is difficult to treat. This is because the retinas in such diseases can be beyond the point of repair on the day of birth. The only way around that would be to use prenatal genetic screening and begin gene therapy in utero.”
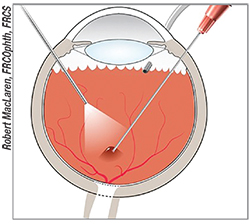 |
| A subretinal gene therapy injection requires the creation of a small detachment. |
Though there are a lot of moving parts to account for in gene therapy, one of the key elements is the vector that’s used to transport the gene to the desired location. The vector that’s currently used in the overwhelming majority of gene therapy studies is adeno-associated virus-2, though researchers say future vectors might bring different benefits that AAV-2 can’t.
To hear it described by Prof. Elizabeth Rakoczy, PhD, director of the molecular ophthalmology department at the University of Western Australia/Lions Eye Institute, the characteristics of AAV-2 make it almost perfectly suited for gene therapy: “At the moment, there are about five different eye diseases being studied in clinical trials by approximately 10 groups, and all of them use a vector that’s recombinant AAV-2, a general vector that enters into all the eye’s cell types,” she says. “The reason for this is that AAV-2 is naturally occurring, is non-toxic in humans—at most causing mild flu-like symptoms—and 80 percent of humans are carriers of it already. Using a virus like this as the basis for your vector is very reassuring, because it has obvious safety benefits.” Prof. Rakoczy is also the inventor of Avalanche Biotechnologies’ gene therapy agent AVA-101 for wet AMD.
Despite AAV-2’s popularity, Prof. Rakoczy says she envisions a future where better vectors emerge. “We need more efficient promoters,” she says, referring to the mechanism of the vector that regulates how much of the desired protein is produced in gene therapy. “Many of the present gene therapy approaches fall short of producing sufficient amounts of biologically active proteins. In many cases, we don’t see the desired improvement in the patient because even though the protein is produced, it’s not in a high enough concentration. That’s why the promoter is essential.
“If you really want to look into the future, ideally we’d like to have vectors that are taken up by the target diseased cell with high efficiency and specificity, and switch on automatically when the target protein drops below normal,” Prof. Rakoczy adds. “These vectors, in combination with the right promoter, would provide the most proficient biofactory tool set against genetically inherited and complex diseases.”
Dr. MacLaren sees vectors as one of the keys to improving gene therapy in the coming couple of years. “There are new variants of the AAV being developed that can be even more efficacious than current ones,” he says. “A more efficient virus, for example, means that you can reduce the dose of the virus and still get the same therapeutic effect, and you really need to get those below a certain threshold to deliver the virus without any immune problems or other issues. In the past few years we’ve actually achieved that goal and now have very efficient viral vectors. We also understand more about the production process, so it’s possible to scale up the AAV to good manufacturing process level—or commercial-grade production levels so it can be made for FDA approval. It’s all well and good making a virus in your lab and using it for research, but to get it approved for general use in patients you need to have a reproducible GMP that’s acceptable to the FDA. We’re now able to achieve that.”
Dr. Bennett says the development of new viral vectors is one of the areas of gene therapy that’s moving quickly. “Researchers are continually modifying the toolkit,” she says. “They’re adding additional attractive features to the vectors by engineering the viral capsids themselves and by taking advantage of evolutionary approaches to evolve these capsids to have attractive features.” The last approach, known as directed evolution, sounds almost like science fiction: Researchers create viruses in a lab in a way that maximizes their diversity, and then promote the evolution of viruses that have the traits that they want through a sort of artificial natural selection. One team of researchers was able to engineer a variety of AAV that delivered its gene cargo in a widespread fashion to the outer retina and rescue disease phenotypes of X-linked retinoschisis and LCA in mice. It also enabled transduction of primate photoreceptors from the vitreous.1
Researchers say one area in which vectors will need to improve in the coming years is very basic, yet very important: their size. “Currently, one of the biggest limitations is the size of the gene that can be packaged within an AAV,” Dr. Bennett says. “I think it would be great if there were further developments that allowed these capsids to package larger amounts of cargo. For instance, if we could double the size of what could be packaged inside an AAV, that would be wonderful. There are either large genes that you just can’t fit into the vectors, or regulatory sequences that we can’t use in the vector to limit the expression of specific cell types because those regulatory sequences are too large to fit inside the vector.
“This size constraint limits the treatment in Stargardt’s, for example,” Dr. Bennett adds. “Stargardt’s therapy is being approached using a virus called lentivirus. Lentivirus barely fits that transgene, but doesn’t allow the inclusion of the extended regulatory sequence that could potentially provide even better results. And, in some circumstances, lentivirus isn’t the best for targeting photoreceptors because it doesn’t target them that efficiently.”
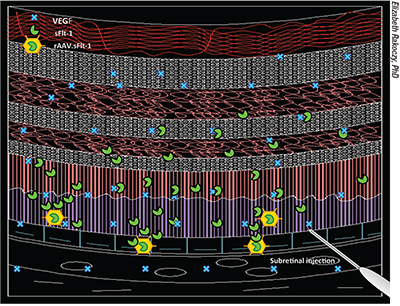 |
| In one iteration of gene therapy for treating wet age-related macular degeneration, rAAV.sFlt-1 is injected beneath the retina, turning cells into biofactories that produce the antiangiogenic agent sFLT-1 to reestablish the normal balance between angiogenic (vascular endothelial growth factor) and antiangiogenic (sFLT-1) factors in the retina. |
In the coming years, surgeons will solidify their route of delivery of the vectors. They now have two approaches to work with—intravitreal and subretinal—each with its own pros and cons.
The main benefit of intravitreal delivery is that it’s technically easier to do: The surgeon injects the dosage of vector right into the vitreous. The drawback is that it may not get the vectors to the site the surgeon wants. Subretinal delivery is more challenging, since it involves injecting into the subretinal space and inducing a small detachment, but it gets the vectors right to their site of action. “I’m a little more skeptical of intravitreal delivery than some other people,” says Dr. Bennett. “I think it could theoretically be used to treat the fovea, but not much more than that. Maybe diseases that cause distinct foveal problems, such as achromotopsia, could be targeted most efficiently with it. The other challenge with an intravitreal route is that in order to get to the site you need high doses. Those high doses can cause inflammation, which would limit the benefit one might get if one were to use them. That’s my main concern: the toxicity vs. efficacy trade-off.”
Prof. Rakoczy thinks this inflammation is a key issue. “If I were to look into the future of gene therapy, I believe subretinal delivery would remain the delivery method of choice,” she says. “It’s been proven that with intravitreal delivery you activate the immune system, so there might be an immune reaction that removes the vector. With subretinal injection, however, you’re injecting into an immune-privileged space, and it seems that this fact is much more powerful than we think. It’s been shown by Jean Bennett’s work that, following a subretinal injection, you can freely inject into the second eye without rejection. In our own work, we’ve found that when you inject into the subretinal space, patients who already had been exposed to AAV2 and have neutralizing antibodies present in their blood actually responded better to the treatment than the average patient.”
Dr. Bennett says, however, that it’s possible intravitreal injection might be useful for the so-called generic gene therapy approaches, where the clinician can treat multiple diseases with the same reagent. “Intravitreal delivery might be useful for the delivery of secretable proteins such as neurotrophic factors or antioxidative stress factors to generate a generic strategy for treating retinal diseases,” Dr. Bennett says, “Delivering neurotrophic factor would make the cells healthier and keep them alive longer. That would be beneficial in many diseases because many are slowly progressive. If you could slow down cell death and allow those cells to live 10 years longer than they normally would have, that would be very meaningful. The same rationale would extend to using antioxidative stress approaches, because there’s a huge amount of oxygen exposure in the outer retina because it’s both exposed to light and has one of the highest blood-flow rates in the body. Finally, one of the more recent findings is that some retinal cells may die in these diseases because they’re not getting enough metabolic support—in other words, they’re not getting fed enough. Researchers are exploring approaches that may provide more nutrients, sugar and energy to these cells to allow them to function better. This could potentially be delivered in a generic approach.”
Another generic option, highlighted by Dr. MacLaren, is the field known as optogenetics, or using gene therapy to switch on a gene that makes a cell light-sensitive, no matter what the genetic cause of the blindness. “It has nothing to do with replacing a normal gene,” he explains, “but is quite often explored with abnormal genes that are present in other life forms that have a light-sensitive protein. It might have application in AMD to make the macula more light-sensitive.”
Prof. Rakoczy thinks there may be improved instrumentation for the injection in five years, as well, assuming a gene therapy approach is approved. “In the lab, we do subretinal injections in the eyes of mice, which are much smaller than the human eye at 2 mm in diameter,” she explains. “To do this, we use a stereotactic system, which means it doesn’t depend so much on the ability of the person doing the injection.” A stereotactic system is a partially automated system that uses computer-generated, three-dimensional coordinates to locate the area in the eye that needs to be injected. The needle’s drive mechanism is fixed to the operating table. When the surgeon activates the mechanism, the needle is mechanically driven into the eye using a screw system while monitoring the depth of penetration on the microscope. “Based on this simple approach, I foresee a more automated system such as this being used, since it’s less dependent on the manual handling of the needle by the surgeon, though some would not agree that it’s better,” Prof. Rakoczy says. “Some might argue that, since in humans the injection is performed under local anesthesia, the surgeon doesn’t have the ability to correct for movement with a stereotactic system as a surgeon might.”
Genetic Testing
Experts say that, though it doesn’t treat disease in the way gene therapy does, genetic testing will evolve in parallel with treatment, since most gene therapy is targeted at a specific gene mutation or other genetic issue.
“For successful gene therapy in the future, we’ll need better genetic testing so that we can test the genes in patients with retinitis pigmentosa or any inherited disease,” says Dr. MacLaren. “Then, we’ll find mutations and know exactly where they are, because you can’t plan a gene therapy program without knowing which gene it is you’re going to create. Going back 10 years, we had only diagnosed a fraction of the genes that cause RP in our patients. Now, with next-gen sequencing, we’re getting 70 to 80 percent of the genes. So, immediately, the pool of potential patients is orders of magnitude higher than it was in previous years.” Dr. MacLaren envisions a future where, as more diseases are diagnosed, physicians can add more mutations to a computerized database so that they’re caught even while looking for something else. “For instance, a patient may go to his cardiologist and get gene testing,” he says. “The heart doctor might look at the test results and say, ‘Hang on, you’ve got an RP gene here,’ and then refer him to us. We can then initiate a treatment possibly 10 years before he would even have been aware of the disease.”
Though Prof. Rakoczy notes that there will be challenges with gene therapy, she’s sure that it will come to fruition, and it will be a blockbuster. “The change in the pharmaceutical industry is coming,” she says. “Within five years, we will see one of the vectors now in trial on the market. The appearance of vector-based ‘biofactories’ will require a new business model for the pharmaceutical industry. This is because now the drug makers get money from every single pill you put in your mouth. But, when you use gene therapy vectors for treatment, once the biofactory is delivered into the body it takes over treating the patient. Thus, at least in theory, they can only sell the product once for every patient. On the other hand, the cost to develop the system is huge, so even though it will be beneficial to patients and cheaper for the government/health-care system, we don’t have a model of how to fund it. Even so, in the future gene therapy will be used more widely for complex diseases such as cancer, heart disease, diabetes and retinal disease. That’s what’s making patients and governments so excited.” REVIEW
Dr. Bennett is the scientific co-founder of and advisor to Spark Therapeutics. Dr. Rakoczy is a consultant to Avalanche. Dr. MacLaren is the academic founder of NightstaRx, a company developing gene therapy treatments.