Dr. Stonecipher points out that topography-guided treatment has been around for a long time. “It got through the FDA with flying colors,” he notes. “In fact, it produced some of the best data we’ve ever seen. In the FDA trial the treatments reduced visual symptoms such as glare, halos and starburst. They improved BCVA; about 33 percent of patients actually saw better than they did before surgery.”
Of course, as with any new technology, there is much to learn in order to avoid pitfalls and get great results. Here, Dr. Stonecipher and two other surgeons well-acquainted with topography-guided ablation discuss how this procedure differs from wavefront-guided and wavefront-optimized ablation; how to use this technology most effectively when treating virgin eyes; how to decide whether a given patient will benefit from this type of treatment; what you need to know if you attempt off-label usage; and offer a list of strategies to help ensure that your patients have the best possible outcomes.
The Topography Advantage
To make the best use of this technology it’s important to understand the differences between wavefront-guided treatment, wavefront-optimized treatment and topography-guided treatment. “Wavefront-guided treatment treats the refractive error, the aberrations, the whole eye from tear film to retina,” says Dr. Stonecipher. “Wavefront-guided takes in all of the imperfections of the optical system and puts them into the treatment profile. In contrast, wavefront-optimized ablation treats sphere and cylinder with an algorithm that was developed to minimize the problems we were inducing with our original laser vision nomograms. Topography-guided ablation, on the other hand, treats the problems being caused by corneal irregularities. In the majority of cases, the cornea is the source of most of the refractive problems—with the notable exception of cataract.”
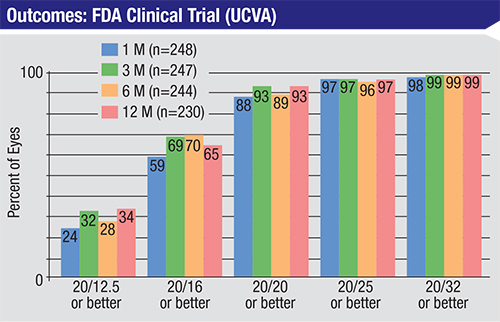 |
| In an FDA clinical trial, topography-guided LASIK significantly reduced light sensitivity, nighttime driving difficulty, reading difficulty and glare. At 12 months, 40.4 percent gained one or more lines of BSCVA; 13.5 percent gained two or more lines of BSCVA.7 |
“One very important aspect of topography-guided treatments is that you’re always making the cornea more regular, and that’s never a bad thing,” adds Arthur Cummings, FRCS, MD, a consultant ophthalmologist at Wellington Eye Clinic in Dublin, Ireland. “With wavefront-guided treatments, it’s possible that you’re creating aberrations on the cornea to offset aberrations that are inside the eye, and that’s obviously not a good thing. You don’t want to punish the cornea for the sins of the lens.”
The “20-unhappy” Dilemma
One of the key issues for American surgeons finally getting access to this technology is that the Food and Drug Administration has only approved its use on some eyes with myopia and/or astigmatism.
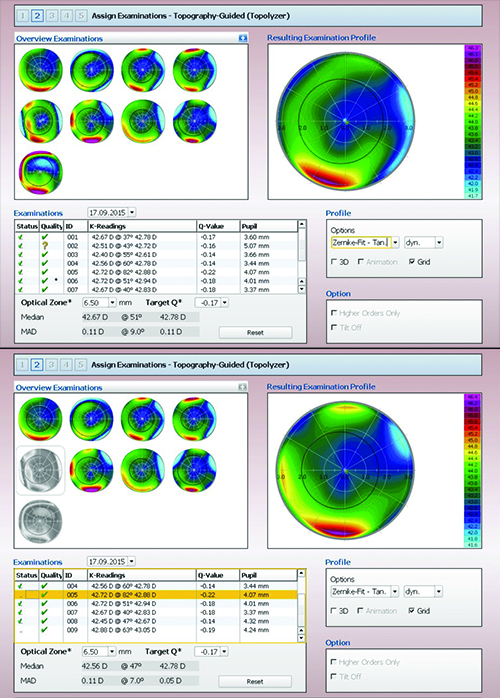 |
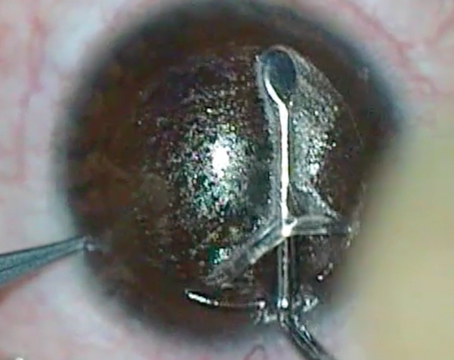
| Although the Contoura software helps to analyze the reliability of the scans on which the ablation may be based (see boxes below scans), the surgeon ultimately decides which to eliminate. Here, the surgeon has chosen to eliminate scans #5 and #9, resulting in a visible change in the profile on which the ablation will be based (bottom screen). |
“Topography-guided customized ablation treatment, or T-CAT, is indicated for myopia up to -9 D, with up to 3 D of cylinder,” notes Dr. Stonecipher. “This technology reduces the induction of aberrations that a conventional treatment, even a wavefront-optimized platform, can produce. It’s great for patients who haven’t had previous surgery and have normal but asymmetric corneal topography. It’s also effective for correcting decentration and enlarging optical zones in patients that have had previous refractive surgery, outside the United States. However, that’s an off-label use of this technology in the United States and not recommended at this time.”
Dr. Cummings says that previously (in his practice located outside the United States) if a patient had no particular complaints, the surgeon would perform a wavefront-optimized procedure. “If the patient did have complaints, we’d try a wavefront-guided procedure, as long as we were able to obtain good data,” he says. “If we couldn’t get good data, we’d do a topography-guided treatment.
“Then, about 18 months ago, the FDA approval for topography-guided LASIK was granted, so Alcon asked a couple of users in Europe to see what they could do with this technology as a first-time treatment,” he continues. “As a result, we started using it for that purpose more frequently. Our WaveLight EX500 laser is linked to an intranet called WaveNet, and we found that it hardly took any extra time to plan a topography-guided treatment. So we started doing more and more topography-guided procedures, and the results were very good.”
“The controversy,” notes Dr. Stonecipher, “revolves around the fact that outside the United States topography-guided laser has always been the panacea for treating the patients who previously underwent RK, PRK or LASIK and ended up dissatisfied with their vision. A lot of American surgeons are talking about finally being able to treat those patients, to fix their corneas and finally make them happy. But for novice users, that is not the way to go. The reason is that normalizing the cornea can leave some patients with a spherical error of -1 or +1, or worse. If the previous refractive surgery left the patient with a very thin cornea, you won’t be able to correct the error you’ve introduced.
“This is why it’s very important for the surgeon just beginning to use this technology to start off with easy cases,” he says. “Start off by treating the -1 and -2 D patients and see how the treatment affects the cornea. Using topography-guided treatments definitely requires changing your nomogram, because smoothing the cornea can induce myopia, hyperopia and even astigmatism.”
Using the Technology
“This technology has changed the way I evaluate patients,” notes Dr. Stonecipher. “Everyone coming in gets a CustomVue wavefront analysis, a Contoura topographic analysis and a Pentacam. We look at all of those to see whether the patient needs LASIK and if so, whether the eye has the anatomy to allow it. Then, we determine what type of platform and treatment the patient should get: wavefront-optimized, Contoura or wavefront-guided. Of course, we also have to make sure the patient doesn’t have ocular surface disease or other issues that would affect the decision.
“There are several key steps to using this technology effectively,” he continues. “First, you have to collect good data. If you can’t get good images, you shouldn’t use it. That’s why topography-guided treatments are a challenge in people with unusual anatomy, ocular surface disease, long eyelashes or deep-set eyes. You don’t want to treat noise. You also need a certain amount of applied area to treat.
“Second, you have to be able to judge which images you’ve collected are good and which aren’t,” he says. “Today, the software helps with this, but you still have to be able to make good judgment calls about which to include in the data pool and which to exclude. Do you have enough mires? Does the eye have ocular surface disease? Are you covering enough area? Remember that this is a very large treatment—close to 9 mm, rather than 6 mm. You’ll need to look at what’s called the raw sagittal data to determine whether a map is good or bad, which is a new concept for laser vision correction. You’ll also need to compare the pictures to see if one or two don’t resemble the others, indicating exam variability. And of course you’ll have to have a good ocular surface to get good diagnostics.
“Third, you want to isolate and look at the part of the ablation that’s aimed at normalizing the cornea, rather than correcting spherical or astigmatic error,” he says. “What is the pure amount of topographic change that you’re going to cause, separate from the refractive error you’re correcting? How will this affect your overall treatment, your nomogram? Will this topographic change make the patient more myopic or hyperopic, and if so, by how much?”
Dr. Cummings explains how to do this. “The instrument can show you exactly what parts of the topography it will be correcting, and the depth of tissue involved,” he says. “If you plan a wavefront-optimized procedure and set it for -2 D, the screen should show a map with about 32 µm of ablation depth. If you then plan a topography-guided procedure on the same case, the ablation depth will likely be a little more than that [because of the additional topographic ablation]. Now, if you change the refractive component to zero or plano, there will still be something on the screen. We call this the higher-order ablation profile; the screen is showing you what it’s going to do to regularize the cornea. The aberrations showing on the screen now are only 2, 3 or 4 µm deep, but removing them is exactly what will treat the topographic issues.”
Dr. Cummings says that in a virgin eye, the wavefront-optimized ablation depth should be close to the topography-guided ablation depth. “When treating an eye that previously underwent refractive surgery, the topography-guided plan might indicate that it will ablate 45 µm instead of 32 µm,” he says. “However, in a virgin eye the ablation depths should be pretty similar between the two treatment options. So, when you’re just starting to do topography-guided procedures on virgin eyes, make sure that your wavefront-optimized ablation depth is close to the topography-guided ablation depth. That will help you avoid making any big mistakes.”
Dr. Reinstein agrees. “If the ablation amounts are very close, you’re in good shape,” he says. “For example, if they disagree by 2 µm, you’re talking about one-seventh of a diopter difference, which is clinically insignificant. In this situation, I would go with the topography-guided treatment; the refractive difference will be tiny, and the cornea will be less aberrated. If the difference between the two ablation depths is 5 or 6 µm or more, you’re venturing into the territory where you might need to compensate for the refractive effect of the topography-guided procedure. If you’re not ready to deal with that, you might want to opt for the traditional procedure until you understand the process better.”
Getting the Details Right
Dr. Cummings explains how his practice uses this technology to treat primary procedure patients. “To do a primary procedure using Contoura, we first identify the patient as someone who has some corneal irregularity,” he says. “Once we begin the process, the instrument takes eight Topolyzer maps and validates them as being either acceptable or not acceptable. [The Topolyzer integrates non-contact topography, keratometry and pupilometry.] There are two boxes on the Topolyzer scans. If they’re both green, that’s perfect. If one’s green and the other is red, you can still use this scan by manually exporting it, but it’s not going to be good enough for key things such as cyclorotational control. Fortunately, you don’t need all eight scans. As long as you have a couple of scans that have two green boxes, the software will predict which one of those has the very best data, and that map will drive the cyclorotational control.”
Dr. Reinstein agrees that the most important part of the treatment is obtaining a high-quality topography scan. He recommends doing the following:
• Check that the exam was properly focused.
• Confirm that the mires are smooth and regular. “If they are irregular, use lubricating drops and repeat the exam,” he says.
• Select the exam with the largest area of continuous data. “Pay particular attention to the superior region, as data acquisition can often be limited by the eyelid,” he notes. “Remember that the ablation algorithm cannot differentiate between a true irregularity and an artifact in the data.”
• Confirm that the scans have been well-centered—i.e., that the patient was looking at the fixation target. “Centration is the most important part of the ablation profile calculation,” says Dr. Reinstein. “The profile should be calculated centered on the corneal vertex—i.e., the center of the mires on the topography scan—not on the entrance pupil center. And since the ablation profile has been generated centered on the corneal vertex, the ablation should be performed on the corneal vertex. Some systems have the corneal vertex location integrated into the ablation profile, but if not, then the surgeon should manually center the ablation on the coaxially sighted corneal light reflex.”
“Cyclorotation is a very important factor in topography-guided ablations—much more so than in standard ablations,” adds Dr. Cummings. “Think of it this way: If you have a jigsaw puzzle on the table with one piece missing, and you have that piece in your hand and your registration is perfect, you can place that piece exactly where it belongs. The surface will be smooth and intact. But if your registration is slightly off in terms of rotation, the piece won’t fit into the slot. You’ll end up making the surface a lot less regular. So it’s crucial to make sure your data is good, the patient is well-aligned and your eye trackers are functioning well.”
Going Off-label
As noted, many American surgeons are anxious to try using this technology to treat 20-unhappy patients, even though this is not part of the FDA approval. The main reason caution is called for is that although topography-guided ablation is designed to improve the corneal surface, it can alter the refraction. “Because you’re correcting aberrations when you change the cornea, whatever you do is going to have a refractive effect,” explains Dr. Cummings. “The refractive change can be quite dramatic when an eye has serious corneal aberrations. If your patient had a procedure 10 years ago to treat a refractive error of -10 D using a broad-beam laser with a less-than-ideal ablation profile, maybe he ended up being -2 D, but he’ll have a lot of spherical aberration. If you refract that patient in the dark, you may find he measures -5 D instead of -2 D.
“If you now do a topography-guided treatment that does nothing more than expand the optical zone, that patient is probably going to change from -2 D to -5 D,” he continues. “That’s why the key to doing topography-guided procedures on seriously aberrated eyes is to get to the point at which you can start predicting what that refractive effect is going to be. Once you can predict that, you can compensate for it. Then the cornea will become more regular and the refraction will be exactly what you intended. You’ll start getting results like those in the FDA study, where night vision was significantly improved and glare was significantly reduced.”
| To Treat or Not to Treat? |
| One of the challenges of this technology is judging whether correcting the corneal surface will make a difference to the patient. “Right now, making this call is a little bit more art than science,” says Karl Stonecipher, MD, medical director of TLC Laser Eye Centers in Greensboro, N.C. “If a patient’s topography is totally normal and symmetric, the benefit of treating with Contoura is simple: You’ll probably have a larger optical zone. This could matter in some patients. But in the clinical FDA trials, there wasn’t a number that told you a given patient would do better with Contoura than he would with wavefront-optimized.” Dr. Stonecipher notes that this isn’t this case when deciding whether to use wavefront-guided ablation. “A study I conducted with Guy Kezirian, MD,” he says, “showed that if an eye has preoperative root-mean-square higher-order aberrations greater than 0.4 µm, patients got better results with wavefront-guided treatment than with wavefront-optimized.6 But we don’t have a study that tells us which patients will benefit from topography-guided ablation yet.” Dr. Stonecipher observes that this has resulted in a wide range of different surgeon choices about how many patients should receive a topographic treatment. “Some surgeons are treating every patient with Contoura, but many other surgeons are not,” he says. “My advice is to start off slowly. Don’t try to do 99 percent of your patients with this technology at the outset. There are many nuances to it. Remember that most problems surgeons encounter with this technology aren’t the result of adulterating the cornea, but the result of causing an unintended spherical error. If patients pay extra to have this treatment, they won’t be happy with an unexpected result—especially one you can’t correct.” Arthur Cummings, FRCS, MD, a consultant ophthalmologist at Wellington Eye Clinic in Dublin, Ireland, notes that appropriate patients often identify themselves. “We’ve found that this technology is a good option when a patient volunteers that he doesn’t see well at night, especially someone who drives at night,” he says. “Another good candidate is any patient we can’t correct to 20/20 or better. Also, sometimes you do a refraction and you see for yourself that there’s a soft endpoint—the patient is not definite about what he’s seeing because his vision is slightly fuzzy. “Basically, we now use this technology whenever we think there’s something preventing the patient from having really acute vision.” Dr. Cummings adds that people are starting to come into his practice asking for the Contoura vision procedure. “Even in an asymptomatic patient you can do a procedure safely that’s going to make his cornea more regular and give him better quality vision,” he notes. “The most important thing is to do it right. Otherwise, you’re going to end up with worse results than you would have gotten with a standard treatment.” —CK |
“My advice to those surgeons would be: If you want to minimize damage and get the best possible result when treating a patient who has previously undergone refractive surgery, start off by doing it as a two-step procedure,” he says. “Tell these patients that this is the way you plan to proceed. Tell them, ‘You have an irregular cornea, but it’s so irregular that if we do a laser vision correction procedure to improve your topography, I have no idea what the impact will be on your prescription, so I can’t compensate for it right away. Instead, I’ll correct the cornea first and make it regular. Then we’ll wait six months. In the interim, you can wear spectacles or contact lenses to address your interim refraction, and because we will have improved your corneal surface, you’ll find that your corrected vision is better than before. Once we get to six months, we’ll measure your refractive error again and do a wavefront-optimized procedure to fix just the refractive component.’ ”
Dr. Cummings points out that the refractive impact of a given treatment has a lot to do with the difference between what the ablation profile indicates will happen at the center versus at the periphery of the ablation zone. “If you’re ablating 15 µm in the center and nothing at the edge, you’re creating 1 D of refractive change,” he explains. “If you’re doing 30 µm in the middle and 15 µm at the edge, you’re also creating 1 D of refractive change. The same is true if you’re ablating 100 µm in the middle and 85 µm at the edge; you’re creating 1 D of refractive change. These are some of the things you’ll learn to think about when predicting the impact of smoothing the cornea.”
Dr. Cummings explains that dividing the procedure into two parts allows you to practice predicting the refractive impact of the corneal smoothing. “Every time you do this procedure, look at the higher-order ablation profile,” he says. “Enter ‘zero refraction’ into the portal software; then look carefully at the digital display showing exactly what the laser will be doing to the cornea to regularize it. You may think to yourself, ‘This looks a bit like a hyperopic correction. It’s changing nothing in the middle but ablating 15 µm in a doughnut shape around the edges. That looks like a 1 D hyperopic correction.’ So you make a note in your file saying you think this procedure will cause a 1-D hyperopic correction. If the patient is currently -2 D, you’re predicting that this patient will be at -3 D when you review his refraction in six months time.”
Dr. Cummings says it might take as few as 10 cases to become good at predicting the refractive effect of smoothing the cornea—if you attend a course on it and spend some time talking to other surgeons who have mastered it. “One reason taking a course is so important is that there are a number of ways to calculate the impact of corneal smoothing, and you can learn them there,” he says. “You may not be an authority in 10 cases, even if you take a course and share other surgeons’ experience, but you’ll be able to manage the more complicated cases quite well.
“To tell the truth, if all you want to do is learn to compensate for the smoothing, you don’t even have to do 10 cases; you just have to understand the principle,” he says. “You can learn a lot just from looking at patient files. But doing the treatments, taking a course, working with other surgeons and seeing how your predictions turn out is what will give you confidence. Eventually you’ll learn from experience to predict what the refractive change is going to be. In the meantime, doing the surgery in two stages is good for patient expectations, it helps you gain experience, and you’re not going to hurt anyone.”
Strategies for Success
Surgeons offer these bits of advice to ensure your outcomes are everything you’d like them to be:
• Start with easy, lower-diopter cases so you can develop your nomogram. “Being part of the FDA clinical trial gave me a chance to develop my nomogram,” says Dr. Stonecipher. “A surgeon’s biggest fear is not having a nomogram. What numbers do I put in for the first patient? That’s why I say to everybody, begin by treating the -1 D and -2-D cases. Work your way up until you feel comfortable with the technology and your results are solid. Because if you go out and treat a -10 on the first day and you make a 10-percent error, you’ll be a diopter off. That’s the difference between 20/40 and 20/15. But if you make a 10 percent error in a -1 D case, the error will be almost negligible. So your first patients should be less than 4 D with probably less than 1.5 D of cylinder, until you get comfortable with the technology and understand it. That way everybody benefits. You won’t be stressed out, and your patients will get great results.”
• Focus on the diagnosis. “Successful use of topography-guided ablation depends on understanding the cause of the visual symptoms,” says Dr. Reinstein. “Always consider how the ablation profile will change the topography and ask yourself if this makes sense in relation to your diagnosis.”
• Pay attention to the details. “To get a great result you need to pay attention to detail—more so than with a standard procedure,” says Dr. Cummings. “In a standard procedure the ablation profile is symmetrical; you can’t really make too many mistakes. But when the ablation is not symmetrical, you have to make sure the treatment is perfectly overlaid on the areas you want to treat. Especially when treating complicated eyes, there’s no question that you can make things worse if you’re not careful.”
• Remember that good data is crucial. “Results of topography-guided treatments will only be as good as the quality of the topography data,” says Dr. Reinstein.
• If you’re considering using topography-guided ablations for all of your primary cases, consider measuring both corneal and whole-eye aberrations. “Topography-guided ablations are restricted to addressing irregularities on the front corneal surface, so a wavefront-guided treatment would be more appropriate if the majority of the irregularities were inside the eye,” says Dr. Reinstein. “This means that it’s important to measure both corneal and whole-eye aberrations if you are considering using topography-guided ablations for all of your primary cases. If you only measure the topography and regularize this, you may inadvertently uncover internal aberrations that the cornea was compensating for.”
Dr. Reinstein says he rarely uses wavefront- or topography-guided ablations in primary surgery cases. (He prefers to use a wavefront-optimized ablation profile, unless there are significant corneal or whole-eye aberrations.) “The reason is that a primary LASIK treatment induces more aberrations than were present before the surgery in the vast majority of patients,” he explains. “Therefore, it’s more important to focus on controlling the induction of aberrations rather than trying to correct the nascent aberrations, which patients are neurally adapted to. Removing those may not always be a good idea.”
He notes that some surgeons do claim to use this technology on all of their patients, and some report improved outcomes. “This could simply be because the base profile of the topography-guided system is better than the standard profile for the laser system they’re using,” he says. “For example, the topography-guided system might include an improved aspheric/peripheral energy correction function, relative to the currently used wavefront-guided or wavefront-optimized profile. It would not be surprising if this achieved better results than the standard profile.”
• Don’t alter the Q-value. One of the things a topography-guided treatment allows the surgeon to do is alter the Q-value, or sphericity, of the cornea. “In most cases, when you’re doing a primary procedure on someone’s eye, all the patient wants to do is see better,” says Dr. Cummings. “In that situation you don’t want to alter the Q-value. In a virgin eye, that’s the Q-value the patient has always had. His brain is used to it and has learned to function well with it. In fact, one of the advantages of topography-guided treatments is that they tend to cause less of a change in Q-value than wavefront-guided or optimized treatments.
“When you load the data, the laser will tell you what the corneal asphericity is,” he continues. “It will give you a Q-value such as -0.3. You want to program the ablation to keep the same Q-value. If you do a standard -3-D wavefront correction, where you’re ablating 45 µm in the center and nothing at the edge, the treatment is going to induce some spherical aberration. If your Q-value before the procedure was -0.3, postoperatively it will probably be -0.2 or -0.15. But when using topography-guided ablation, you can target a postoperative Q-value of -0.3. This will help change the asphericity as little as possible. So, when setting up an ablation, make sure your target Q is the same as your presenting Q.”
| “Your first patients should be less than 4 D with probably less than 1.5 D of cylinder until you get comfortable with the technology and understand it.” —Karl Stonecipher, MD |
• Keep “tilt” turned off. “Many American surgeons will be using the Alcon Allegretto 400 Hz Eye Q to perform topographic treatments,” says Dr. Cummings. “That laser defaults to the setting ‘tilt on.’ Tilt is a lower-order aberration that compensates for alignment; another way to think of it is as a prismatic correction. When tilt is turned on a procedure typically uses significantly more tissue than when tilt is turned off. So, if you’re using the 400-Hz Eye Q, go to that box and unclick it. You don’t want tilt as part of your ablation.” (Dr. Cummings adds that, in contrast, the 500-Hz laser defaults to ‘tilt off.’)
• Educate staff and colleagues about this technology, not just patients. Dr. Stonecipher says it’s crucial to educate those you work with—both inside and outside your practice—about the nature and advantages of this surgery. “You have to begin by educating your staff so they can answer patient questions,” he says. “At the same time, you have to talk to your affiliates and colleagues and referrals.”
Dr. Stonecipher notes that a surgeon’s colleagues often don’t understand the difference between topography-guided treatments and the other treatments. “If they confuse your patients, the whole point is lost,” he says. “I tell them, this new technology not only improves a patient’s quantity of vision but also quality of vision. It not only improves your spherical outcome, it improves how well you see, day or night, under low illumination and in different contrasts. Both patients with regular but asymmetric topography and unique corneal surface abnormalities can be treated with this technology.
“Of course, you need to make sure your patients understand this as well,” he says. “This is particularly important if you’re charging more for the procedure. For example, I might tell a patient, ‘You can go to a store and buy an off-the-rack suit, but you may really need one that’s tailored for you.’ That’s an analogy patients understand.
“Today,” he adds, “most of our patients are contacting our practice through the Web, so our website has to have this information, too.”
• Don’t combine wavefront and topography-guided ablations. “The wavefront data contains the irregularities from the topography,” Dr. Reinstein points out. “Combining them would mean treating the corneal irregularity twice.”
The Learning Curve
Dr. Reinstein says that the learning curve when undertaking topography-guided ablation is relatively short if you’re treating standard eyes. “This is essentially the same as doing a wavefront-guided treatment,” he says. “You obtain a scan, import it and the software calculates the ablation based on this data. In contrast, the learning curve for treating therapeutic patients is quite long, but this is almost entirely about learning to select the appropriate patients. In other words, it’s the diagnostic process that involves a significant learning curve.”
“Alcon is putting together a group of previous users who participated in the clinical trials, an advisory panel to help train doctors,” notes Dr. Stonecipher. “When you’re being trained you’ll go to Alcon to get the software and unlock Contoura. You’ll be required to do an online assessment. Then a trainer will come and show you how to use it and which patients to choose, and help you get through your first cases. Alcon is doing everything they can to make sure this has a good outcome.”
Dr. Reinstein believes that topography-guided ablation may have an impact on the future success of refractive surgery in general, particularly because of its ability to undo undesirable corneal effects that sometimes accompany laser refractive surgery. “I believe the main reason a majority of people still don’t want to have refractive surgery done is that they’re afraid that if something goes wrong we won’t be able to fix it,” he says. “The truth is, with the currently available custom ablation profiles based on wavefront aberrometry and corneal topography and our understanding of epithelial masking and the therapeutic use of transepithelial phototherapeutic keratectomy, we’re able to repair the majority of irregular corneas. As this message spreads and more surgeons become comfortable diagnosing and treating therapeutic patients, I’m confident that this will be accompanied by an increased public confidence in laser eye surgery as a whole.” REVEW
Dr. Cummings is a consultant to Alcon Laboratories and WaveLight GmbH. Dr. Reinstein is a consultant to Carl Zeiss Meditec. Dr. Stonecipher is a consultant to Alcon.
1. Reinstein DZ, Gobbe M, Archer TJ. Coaxially sighted corneal light reflex versus entrance pupil center centration of moderate to high hyperopic corneal ablations in eyes with small and large angle kappa. J Refract Surg 2013;29:518-525.
2. Chang DH, Waring GO. The subject-fixated coaxially sighted corneal light reflex: A clinical marker for centration of refractive treatments and devices. Am J Ophthalmol 2014;158:863-874 e862.
3. Reinstein DZ, Archer TJ, Gobbe M. Is Topography-guided Ablation Profile Centered on the Corneal Vertex Better Than Wavefront-guided Ablation Profile Centered on the Entrance Pupil? J Refract Surg 2012;28:139-143.
4. Reinstein DZ, Archer TJ, Gobbe M. Combined corneal topography and corneal wavefront data in the treatment of corneal irregularity and refractive error in LASIK or PRK using the Carl Zeiss Meditec MEL80 and CRS Master. J Refract Surg 2009;25:503-515.
5. Reinstein DZ, Archer TJ, Couch D, Schroeder E, Wottke M. A new night vision disturbances parameter and contrast sensitivity as indicators of success in wavefront-guided enhancement. J Refract Surg 2005;21:S535-540.
6. Stonecipher KG, Kezirian, GK. Wavefront-optimized Versus Wavefront-guided LASIK for Myopic Astigmatism with the ALLEGRETTO WAVE: Three-month Results of a Prospective FDA Trial. J Refract Surg 2008;24:4:S424-30.
7. Data available at http://www.accessdata.fda.gov/cdrh_docs/pdf2/P020050S012b.pdf.