| Disease Diagnosis Counts Through May 2015 | |
| • All Macular Degeneration • Exudative Macular Degeneration • Primary Open-angle Glaucoma • Diabetic Retinopathy • Cataract Surgeries | 806,775 229,330 659,243 339,472 857,738 |
“We started planning this project in February 2012,” explains William L. Rich III, MD, American Academy of Ophthalmology’s medical director of health policy and chairman of the executive committee of the IRIS Registry. “We solicited the software vendor and received funding from the board in late 2012. We developed our quality measures and started implementing the software in 2013. Finally, we launched the registry in April 2014, with the goal of serving the needs of 2,200 physicians and 8 million patients by the year 2017.
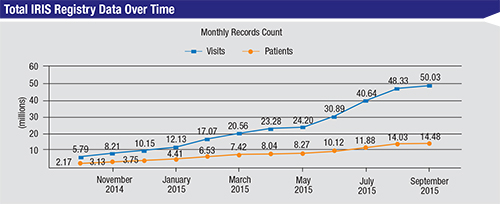
“We surpassed that goal very quickly,” he continues. “We now have data from 10,800 ophthalmologists covering more than 15.3 million patients and more than 54 million office visits. I believe this demonstrates the perceived value of the IRIS Registry by the membership. And it’s worth noting that the registry isn’t supported by any commercial money. All of the funding has come from the Academy’s reserves; it’s the greatest expenditure in our 100-year history.”
Dr. Rich says they’ve already begun assessing the validity of some of the data. “We looked at the data from the first 636,000 cataract surgeries submitted to the IRIS Registry and compared it to the data from a very structured cataract surgery registry in Sweden,” he says. “Our outcomes numbers were within about 2 percent of theirs.”
Of course, any large-scale effort like this—especially one involving mass data collection and electronic movement of information—raises a host of questions. Here, Dr. Rich and Cynthia Mattox, MD, FACS, (vice chair of the Department of Ophthalmology at Tufts University School of Medicine, director of the New England Eye Center Glaucoma & Cataract Service, and chair of the Task Force on Quality Measure Development for the IRIS Registry) discuss the potential benefits of the IRIS Registry and address the concerns that some surgeons have expressed.
Regulatory Requirements
“Right now, the main role of the IRIS Registry is to help doctors comply with Physician Quality Reporting System requirements and the Meaningful Use Clinical Quality Measures,” says Dr. Mattox. “By reporting data through either a cataract measure group or the Registry, doctors are successfully meeting the requirements for the PQRS. That’s only going to get more important as we go forward with the new program that was created in the 2015 Sustainable Growth Rate reform bill—the Medicare Access and Children’s Health Insurance Program Reauthorization Act legislation, or MACRA—which makes things even more complex. As a matter of practice economics, being a participant in the IRIS Registry makes things so much easier. It’s almost impossible to participate in these programs just by reporting on claims anymore.”
“For this to work most efficiently, you have to be using an EHR that’s been connected through the IRIS Registry,” notes Dr. Mattox. “In order to allow the IRIS Registry to capture the data required for PQRS, an extraction software tool connects to your EHR and locates the data needed for the measures. Fortunately, most of the background work that makes this possible has already been done by the IRIS Registry, working with the EHR vendors to map where those pieces of data lie within their databases. About 36 popular EHRs are already mapped for this purpose.
“Of course, there are individual variations in each doctor’s practice,” she continues. “One doctor might document a certain type of data in a certain location; another doctor might document the information somewhere else. For that reason, every participating practice has an IRIS Registry client account manager who works with the doctors to figure out where the data is located within the EHR record. The fact that the IRIS Registry has already done 90 percent of the background work of mapping with the EHR vendors makes it much more efficient to get this working.”
Comparison to Peers
One of the most obvious benefits that can accrue from assembling this type of large-scale data is being able to compare yourself to the rest of the profession—or to compare the doctors within your own practice. “As surgeons, we think we do a good job, but we can still improve by comparing ourselves to our peers and learning from one another,” says Dr. Rich. “When you receive your PQRS results from the federal government, the data is 18 months old. Whatever it says about you, it’s so old that you can’t really act on it. As a result, no one pays much attention to it. With the IRIS Registry, about a month after you do a procedure you’re going to be able to compare your results to a database with more than a million cataracts. That’s pretty powerful, and doctors are already taking advantage of it.
“Basically, physicians are very competitive people,” he continues. “They compete in college, in medical school, during internships and residencies. Their success rate is always being noted and intensely scrutinized, even during their training. So physicians are kind of used to being measured. Eventually, I think pretty much all ophthalmologists will be a part of this. If you really want to improve as a doctor, it’s nice to be able to compare yourself to a big benchmark. A registry lets you do that.”
| The IRIS Registry: A User's Perspective |
| “As far as I know, our practice was one of the first to implement the IRIS Registry,” says John M. Haley, MD, in private practice in Dallas for near 40 years, and a member of the American Academy of Ophthalmology Health Policy Committee. “Start-up was remarkably easy. IRIS is hooked up to your EHR system with help from your IT people. There’s a required mapping process where you communicate with the IRIS group and let them know specifically where you document PRQS measures, such as the presence or absence of DME, in your EHR. All of the measures must be mapped for each doctor, unless everyone in your practice does things exactly the same way. This sounds complicated, but it all goes quickly and easily. Once you’re set up, the IRIS computers work behind the scenes at night; they extract that DME information from your records and give you a check that indicates that you performed the evaluation. “Progress in your practice can easily be followed on your personal dashboard,” he continues. “Any problems that occur can be addressed by IRIS before there’s any risk of failing your PQRS evaluation. You can also evaluate all the partners in your practice using various parameters, although in our practice this has been limited to the PQRS measures. We’re now able to bring all partners up to one standard quickly and easily, thanks to the IRIS monitoring. “Outcome comparison will be important in the future,” he adds. “And, there’s no other practical way to comply with all the quality measures that will determine our bonuses and penalties. Data will be king in the future, and IRIS will allow collection of that data for clinical studies, as well as personal outcomes that determine quality. Using the IRIS Registry is a no-brainer.” —CK |
“If there’s any problem with the data,” she adds, “it may be that the information is not being picked up in a particular location. The client account manager can help to fix that. Sometimes it’s just that a doctor is not remembering to document something, even though he’s doing it. That can prompt a conversation with the doctor to put the necessary documentation in the record.”
“In terms of comparing the doctors within a practice, when you’re in a big group—my practice has 12 doctors, for example—you probably have no idea how each of you does in comparison to the others,” says Dr. Rich. “IRIS will let you make that comparison. Of course, the only person that has access to that information is your practice and you. I know of one practice that did this and discovered that one member of the group, a well-established surgeon with a good reputation, had a complication rate four times higher than the rest of the practice. I don’t know what the group decided to do with that information, but I’m sure they were glad to be aware of it. Of course, this type of information will create some interesting challenges, but they are challenges worth addressing.”
Improving Outcomes
“One of the goals for the IRIS Registry is to help physicians meet federal regulatory requirements for better outcomes,” notes Dr. Rich. “If you ask the question, ‘Do registries really improve outcomes?’ the answer is that they definitely do. That’s been shown incontrovertibly by our peers at the Society of Thoracic Surgery and the American College of Cardiology, both of which have long-standing registries. Or, look at our peers in Sweden. Since they implemented their registry the complication rates in cataract surgery have steadily and continually gone down, both in low-volume and high-volume hospitals. So there’s no doubt about it.”
“Having access to this kind of database definitely raises the bar,” agrees Dr. Mattox. “And in the future, we hope to learn new things about patient care that can be identified within the IRIS Registry data. The time it will take to do this will be far shorter than the time it takes to complete a controlled clinical trial, which can be years. Of course, there will always be a role for randomized clinical trials, but it’s a different mindset. Clinical trials target very specific questions looking for very specific answers. In the IRIS Registry, the sheer volume of data should reveal answers that we may not even have been looking for.
“The first phase of using a registry like the IRIS Registry is measuring and benchmarking and making sure that your practice is up to snuff,” she adds. “But the second phase is taking the data to another level, where we identify serious deficiencies or risk factors that can push care in a different direction.”
Simplifying Clinical Trials
Dr. Rich notes that registry data may help researchers conduct large clinical trials for much less cost than traditional methods. “If you look at the cost of clinical trials in the United States, even a small trial like the Comparison of Age-Related Macular Degeneration Treatments Trial costs more than $10 million over a period of three years,” he says. “Some trials cost more than $100 million. Our society really can’t afford to spend that kind of money. Registry data may provide a way to solve that problem.
| Comparative Rates of Endophthalmitis (April 2014 – May 2015) | ||
| Drug | Injections | Endophthalmitis rate |
| Avastin Lucentis Eylea | 478,381 245,381 103,390 | 0.12% 0.09% 0.12% |
| One of the ways in which registry data can be used is as a means to resolve questions that may impact public policy. Questions regarding the relative risk of endophthalmitis when injecting Avastin, a compounded drug, versus noncompounded drugs such as Lucentis and Eylea were put to rest after looking at the accumulated data for those types of injections. | ||
Dr. Mattox notes that using the IRIS data for clinical trials is still down the road a ways. “However, this will be a huge gold mine for something like that,” she says. “This is real-world longitudinal clinical data from many, many patients—more than 14.2 million unique patients have been captured in IRIS as of early September. Not only could you pull patients from the registry in order to do clinical comparative-effectiveness research, you’ll be able to stratify risk because we’ll have all the associated diagnoses, medications and everything else that you can extract from an electronic record. Already there are interesting analyses being performed on the data, but this is in its infancy. Eventually, this will be an amazing, powerful benefit.”
Other Potential Benefits
Dr. Rich notes several additional ways ophthalmology may benefit from the existence of the IRIS Registry:
• Impacting public policy. Dr. Rich points out that this kind of “big” data can have a serious impact on public policy—and already has in some instances. “In April of this year there was a major debate taking place in Washington, D.C., about when and how compounded drugs, such as anti-VEGF agents, should be injected into the body,” he says. “A committee was formed to make recommendations to the FDA. One member of the committee was associated with a company whose profits might be affected if the injection of non-approved compounded drugs were encouraged. That person basically said that no one in the United States should have a non-FDA approved drug injected into the eye because of the risk of endophthalmitis, despite the safety data from the CATT trial, which suggested that there was equal safety whether the drug was compounded or not.
“In response, George Williams, MD, who is the secretary for federal affairs in the Academy, asked Dr. Flora Lum, AAO vice president of Quality and Data Science, if the IRIS Registry had produced any data relevant to this question,” he continues. “A few days later, Dr. Williams was able to show the committee the data from 827,000 consecutive injections of an anti-VEGF agent into the eye, comparing the endophthalmitis rates between Lucentis, Avastin and Eylea. The difference was 0.03 percent between the drugs. That ended the debate. In the past, addressing a question like this with this level of data would have been nearly impossible.”
• Monitoring the safety and efficacy of drugs and devices. “Remember Vioxx?” asks Dr. Rich. “It took years to figure out the correlation between Vioxx and cardiac events. The FDA is currently attempting this type of monitoring using what they call a ‘Mini-Sentinel Initiative’ where they look at big data from many sources. We think that’s going to play a huge role in the surveillance of ophthalmic devices in the future. Currently, about 60 percent of Class III devices have mandated post-market surveillance by the FDA, but only 6 percent are being monitored by independent registries. The rest are commercial registries formed by the industry. The FDA would certainly prefer a more independent source for that kind of data, so I think there’s a huge opportunity for societies like ours to get involved in device and drug surveillance. That’s one of our plans at the Academy—to develop a practice-based research network that can do both research and device surveillance.”
• Monitoring public health. “Previously, we never really had a large database to look at ophthalmic disease in the United States,” notes Dr. Rich. “We’re now becoming involved with multiple large public-health comparative-effectiveness applications, including an eye disease surveillance project backed by the Centers for Disease Control and Prevention. The IRIS data would be the backbone for those applications.”
• Creating common standards. Dr. Rich says the registry is also hoping to find common ground with the American Board of Ophthalmology. “Becoming board-certified can be laborious,” he notes. “We’re working with the ABO to find ways that board certification can utilize some of the data that’s already being extracted, to minimize the need for picking up charts and filling out forms. We also want to help ensure that the things being measured are meaningful for the board, the profession and the public, which is not necessarily the case now. Ophthalmology boards are appropriately independent from the specialties, and our goals are somewhat different from theirs. Their goal is to protect the public good by developing standards; our goal is to educate and improve the performance of physicians. But if we all used the same metrics for measurement, that would make a lot of sense.”
What if the Data Is Hacked?
“The prospect of the database being hacked is a little scary,” admits Dr. Rich. “However, in contrast to major companies like Target or Blue Cross, there hasn’t been a data breach in any registry, even though a number of them have been around for several years. They all use the highest degree of security available, although of course that’s not a guarantee.”
“As we know, anything can be hacked,” he continues. “It’s something everyone worries about. But hacking your IRIS information from the cloud would have about the same impact as if someone got the information from hacking your credit card database; they would not have access to the linked data on your health. So someone with that data wouldn’t be able to discriminate against you if you have cancer, if you’re a candidate for a job and might have exorbitant costs to insure. Right now, I don’t think we’re of much interest to hackers.”
Dr. Mattox notes that preventing hackers from accessing the registry is a technical issue beyond the expertise of many of the ophthalmologists running it. “FIGmd is the IRIS Registry vendor,” she notes. “Their company has more experience with registries and database-capture than any other company. As I understand it, all of the identifiers and demographics are kept in a completely different server from all of the clinical information,” she says. “So, everything on the clinical side of the aggregate data is de-identified. Within your own practice, if you’re given the rights to look at others in your practice, you can see each physician’s data. However, even doing that requires access permission. From practice to practice or region to region, the setup is much different. You won’t be able to look at the data from Dr. X’s practice down the street.”
Dr. Rich adds that the size of this type of data collection is relative. “This qualifies as ‘big data,’ but not really, really big data, like the type the NSA collects when monitoring people’s phone calls,” he says. “For medicine this is big, but we’re not talking about high-speed collection of unbelievable volumes of billions of data inputs like the security agencies use.”
Could the Data Be Biased?
Some surgeons wonder whether the statistical data captured by the IRIS Registry might be slanted as a result of coming from mostly EHR-using, self-selected practices. Dr. Rich says the quantity of data is so large that he doesn’t think this will be an issue. “For example, we’re going to have data about a couple million intravitreal injections and cataracts,” he says. “Furthermore, we’ve looked carefully at who’s participating in IRIS. The spread of practice sizes and mix of specialties among the participants is the same as in the general membership.
“Of course,” he continues, “using or not using EHR will be a delineating factor, but it’s not clear that that alone will affect the data in any significant way. About 50 percent of ophthalmologists are now using EHR, and the number keeps growing. Some subspecialties, such as pediatric ophthalmologists, may not gain much by implementing EHR, and older ophthalmologists who are near retirement probably won’t want to make a huge change like that. However, of the doctors who are using EHR, 90 percent are on IRIS now. And we think that will increase in the next two years.”
Dr. Mattox notes that even if your practice doesn’t use electronic records, it’s still possible to participate. “It’s extremely difficult to report on the required nine measures for PQRS without an electronic records system,” she admits. “However, in 2014 about 700 doctors who don’t use EHR participated in PQRS by allowing the IRIS Registry to report data for the cataract measure group. This requires reporting on a specific set of measures, but you only have to report them for 20 of your uncomplicated cataract patients. The patients need to be Medicare patients, but you can include nine Medicare Advantage patients. For 2016, we also have approval for a diabetic retinopathy measure group reporting option.”
Other Concerns
• Will the data create legal liability if you have a bad outcome? “There hasn’t been an issue yet with discoverability,” says Dr. Rich. “Going back 20 years or more, there has not been a successful subpoena of a registry chart. Personal charts, yes—those are obviously discoverable. But no one should have access to someone’s comparative effectiveness. I don’t know the rate of endophthalmitis or dropped nuclei of the surgeon down the street. Only he or she knows that, as well as those who are also in that practice. Your comparative success in doing a procedure, no matter what device, drug or approach you use, is not legally discoverable. The IRIS database is not public unless we make it public. It’s owned by the Academy and its members.”
• Won’t this affect workflow in the office? Dr. Rich says it will not. “The Academy formed a registry in 1995 called NEON, the National Eye Outcomes Network,” he explains. “To submit data you had to use a piece of paper, like the SAT test; you filled in little boxes and darkened the circles. NEON was a disaster because it interfered with workflow. Our practice was a beta site, and my staff begged me to stop. With the IRIS Registry, a piece of software sitting on top of your server pulls out the day’s data at night and loads it into the registry. There’s no staff work. The fact that it doesn’t affect workflow is a huge benefit.”
• Could this kind of data cause protocols to become overly restricted? Some surgeons are concerned that once massive amounts of data indicate that one method produces the best results, insurance companies may refuse to reimburse any alternative options—unless, perhaps, the surgeon completes extensive paperwork explaining the reasons for not using the preferred approach.
Dr. Rich believes this is unlikely to become a problem. “As long as you get good results and cause no injuries and follow the norms, I don’t think that’s possible,” he says. “However, if you have a patient with wet macular degeneration and you want to treat that patient with a hot laser like we did in the ’80s and ’90s, while everyone else is now using anti-VEGF drugs, well, you shouldn’t be doing that. If you’re caught, you’ll have a legal problem. But preferring one device or approach over another? I just don’t see that, as long as your outcomes are reasonable.”
| Can Comparisons Be Fair? |
| How surgeons may be compared to other surgeons is a sore point for many physicians. “We’ve been very careful and cognizant of that along the way, in terms of how comparisons are going to be made,” says Cynthia Mattox, MD, FACS, chair of the Task Force on Quality Measure Development for the IRIS Registry. “I would say this: Right now, any profiling done by private payers is all about simply comparing ophthalmologists to other ophthalmologists. There’s no stratification of subspecialists, taking into account that a retina specialist is going to have higher costs than a comprehensive ophthalmologist. There’s no differentiation between ophthalmologists who do surgery and those who don’t do surgery. In other words, private payers are not using the correct peer groups for comparison. That’s a big problem: garbage in, garbage out. “In contrast,” she continues, “something as sophisticated as the IRIS Registry is going to allow us to compare all the retinal specialists, or all the doctors who do a similar number of trabeculectomies, across the country. We’re not there yet, but this is the future. And you’ll get a much more robust comparison of your results or the makeup of the patients within your practice—how many patients are severely afflicted with diabetes compared to other practices, for example. The risk stratification and profiling and meaningful comparisons that can be done using a big database like the IRIS Registry is leaps and bounds beyond what is currently happening in private-payer situations today. This will be far better than what we have now.” Dr. Mattox notes that what is being measured also makes a big difference, and payers are pushing “quality” measurement on ophthalmologists without involving the physicians. “This type of registry, where we design the measures that are of interest to us and look at the questions of interest to us, makes much more sense,” she says. “This will allow us to make proposals to the policy makers, saying, ‘Look, this is something that’s important that needs to be changed.’ They would never identify these things using their systems. “There are always people who are concerned that they will be targeted in some way as a result of this kind of comparison,” she adds. “But in reality, I think the IRIS Registry is going to prove to payers that we are providing exceptional care. We’ll be able to demonstrate that the people who are taking on those more difficult cases have a reason to be more costly providers, just based on their patient demographics and severity. That’s where we’re headed. We want this to help members, not harm them.” —CK |
• Measuring outcomes would provide more meaningful data than comparing doctors’ completion of processes. Insurers tend to measure whether doctors’ are completing the steps in a process instead of measuring treatment outcomes. Dr. Mattox says the IRIS Registry is working toward measuring both. “Most of the PQRS measures are still process measures,” she says. “Those ask: If a patient has a particular condition, are you doing X, Y and Z? In contrast, an outcome measure asks how well your patients are doing after your treatment. There’s a big push toward outcome measures, but we don’t want to get rid of the process measures; they are ophthalmology-specific, and there are practice gaps that relate to them that we need to pay attention to.
“The IRIS Registry is now designated as a clinical-quality data registry, which allows us to develop our own homegrown quality outcome measures,” she continues. “At this point we have 18 approved subspecialty quality measures that are outcome measures. Those outcome measures are optional right now, but it’s possible to choose two outcome measures in order to be a successful participant in the PQRS program in the future.”
Dr. Mattox says measuring outcomes is the direction in which the IRIS Registry is moving. “The ones that are approved are just our first go-round, trying to identify some measures that are easily measured with the data that we know is captured by EHR,” she says. “Eventually they’ll be revised and improved. We’ll have more information in the database that we can pull from to identify the next phase of measures.”
The Wave of the Future?
“The Society of Thoracic Surgeons and the American College of Cardiology were the real pioneers in the creation of medical registries,” notes Dr. Rich. “Today, everyone is realizing that this is a good thing. Medical specialties want to educate their members and improve quality of care, and most of them have decided that this is the best way to do it. In the past two years, they’ve seen the success that the pioneering specialties have had. As a result, registries are proliferating right now. I think soon almost all specialists will have a registry. If they haven’t set one up already, they’re in development or negotiations to do it. I don’t think there’s much downside to participating.
“Right now we’re the largest registry in the United States, and I’m pretty sure in the world,” he adds. “It’s been a lot of work, but we’ve been surprised at the level of acceptance. The vendor we’re working with has had to hire triple the number of staff to handle the uptake. It’s an exciting time.”
Dr. Mattox notes that this is just the beginning. “The measures we’ve developed are rather coarse, and there will be criticism of that,” she says. “But I feel strongly that as we move forward and learn more about data collection and analysis, we’ll be able to refine the measures and identify information that really moves the needle. Right now this is a work in progress. We encourage doctors who have ideas about what we should be measuring, or how we should be measuring it, to get involved and help us.”
Dr. Mattox notes that some fear about unintended consequences surrounding a project like this is to be expected. “However, I think most of that fear is unfounded,” she says. “Whatever we can do to control our own destiny as a profession is better than waiting for our destiny to be determined by people who don’t know anything about ophthalmology. That’s what’s happening now. We’re changing the conversation, and that’s a good thing.” REVIEW
1. Lauer MS, D’Agostino RB Sr. The randomized registry trial--the next disruptive technology in clinical research? N Engl J Med 2013;369:17:1579-81.