Prior to discharge from the Wills Eye Emergency Department, a complete blood count with differential, PT and PTT were reviewed and were unremarkable. The patient was subsequently referred to the Retina Service of Wills Eye Hospital for further workup and treatment.
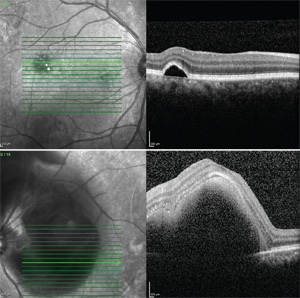
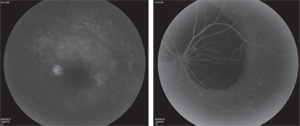
Optical coherence tomography disclosed a macular serous pigment epithelial detachment in the right eye and a large submacular hemorrhage without subretinal fluid in the left eye (See Figure 2). Fluorescein angiography revealed diffuse mottled hyperfluorescence with focal hyperfluorescence localized to the PED in the right eye and diffuse choroidal fluorescein blockage due to subretinal hemorrhage in the left eye.
Although indocyanine green angiography was not performed, the overall clinical picture and diagnostic testing were consistent with a diagnosis of idiopathic polypoidal choroidal vasculopathy (IPCV). Given the degree of submacular hemorrhage, the patient underwent pars plana vitrectomy with injection of subretinal tissue plasminogen activator and intraocular sulfahexafluoride (SF6) gas bubble tamponade. With gas tamponade, inferior displacement of the subretinal hemorrhage was achieved.
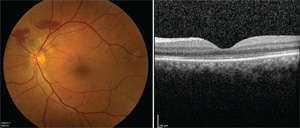
Four months following surgery, the patient experienced a markedly improved anatomic (See Figure 4) and visual outcome with corrected visual acuity of 20/25 in the left eye. Throughout this time, the previously noted extrafoveal PED was monitored and remained stable without intervention in the right eye.
Discussion
|
Aptly described by its name, IPCV is a vascular disease process characterized by lesions produced by a network of branching choroidal vessels with terminal, polyp-like aneurysmal dilations. On ophthalmoscopic examination, this can manifest as orange to red lesions protruding from the choroid into the subretinal space. Patients with IPCV experience multiple, recurrent, serosanguinous PEDs and neurosensory retinal detachment secondary to leakage and/or hemorrhage from the abnormal choroidal vasculature.5 Both the etiology and pathophysiology of the IPCV disease process are still poorly understood.
AMD patients unresponsive to standard therapeutic measures with anti-vascular endothelial growth factor agents are nominees for IPCV workup, especially if the findings are unilateral and patient demographics align. While no universally accepted criteria for definitive diagnosis of IPCV exist, the current gold standard for diagnosis is visualization of polypoidal lesions on indocyanine green angiography.6 ICGA is the preferable angiographic study because IPCV lesions can closely mimic CNV membranes on FA. En face OCT has been shown to be effective in detecting not only subretinal and sub-RPE fluid, but polypoidal lesions as well, making it another potentially helpful diagnostic tool in IPCV.7
Following diagnosis, several factors may dictate the treatment algorithm. Several studies have established that the best visual and anatomic outcomes for PEDs or subretinal fluid have been achieved with use of photodynamic therapy (PDT) coupled with periodic intravitreal injections of anti-VEGF agents.3,8 In a study by Kaoruko Tomita and colleagues, it was shown that PDT used in combination with ranibizumab led to significant visual recovery in eyes previously untreated with PDT, but failed to show the same benefits in eyes that had demonstrated recurrence after previous PDT treatment.9 More recent data on three-year visual outcomes of treatment-naïve patients comparing treatment with PDT and intravitreal bevacizumab (double therapy) to PDT, intravitreal bevacizumab and subtenon triamcinolone acetonide injections (triple therapy) showed superiority of the triple therapy treatment regimen.10
While not necessarily applicable to IPCV, the Submacular Surgery Trial examined the utility of submacular surgery versus laser photocoagulation in the treatment of recurrent subfoveal neovascular lesions in AMD and found no difference in visual outcomes for patients who underwent submacular surgery or laser photocoagulation.11 However, in the setting of massive subretinal hemorrhage associated with AMD and its variant IPCV, surgical treatment options predominate. A multicenter interventional case series highlighted the utility of PPV, subretinal injection of tPA, and gas bubble tamponade for displacement of submacular hemorrhage in AMD with associated improved visual outcomes.12 In a study examining the use of intravitreal tPA injection and pneumatic displacement for treatment of submacular hemorrhage, the hemorrhage attributed to IPCV was completely displaced in all 11 study eyes.13 Interestingly, it was further noted that eyes with IPCV treated in this manner had better visual outcomes than eyes where submacular hemorrhage was attributed to “classic” AMD. A case series from Fumio Shiraga and colleagues reported improved visual outcomes with multiple surgical treatments including submacular removal of neovascular membranes, PPV combined with subretinal tPA, or SF6 gas bubble tamponade alone.14
Despite its similarities to AMD, the diagnosis and management of IPCV has its own challenges and the search for optimal multimodal treatment regimens continues. With PDT, anti-VEGF agents and numerous surgical interventions at the ophthalmologist’s disposal, the prognosis for most IPCV patients remains generally good. However, patients with excellent visual outcomes, as in our case, should be continually monitored for pigment epithelial hyperplasia, atrophic degeneration and subretinal fibrosis.15 REVIEW
The authors would like to thank Joseph Maguire, MD, of the Wills Retina Service for his time and assistance in preparing this case report.
1. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic choroidal vasculopathy. Retina 1990;10:1–8.
2. Yannuzzi LA, Ciardella A, Spaide RF, Rabb M, Freund KB, Orlock DA. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol 1997;115:478-85.
3. Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003;121:1392-6.
4. Davis SJ, Lauer AK, Flaxel CJ. Polypoidal Choroidal Vasculopathy in White Patients. Retina (Philadelphia, Pa). 2014. Jun 20. [Epub ahead of print] PubMed PMID: 24978430.
5. Oluleye Ts, Babalola Y. Pattern of presentation of idiopathic polypoidal choroidal vasculopathy in Ibadan, Sub-Saharan Africa. Clin Ophthalmol 2013;7:1373-6.
6. Cheung CM, Lai TY, Chen SJ, et al. Understanding indocyanine green angiography in polypoidal choroidal vasculopathy: The Group Experience With Digital Fundus Photography and Confocal Scanning Laser Ophthalmoscopy. Retina 2014 Jul 28. [Epub ahead of print]
7. Kameda T, Tsujikawa A, Otani A, et al. Polypoidal choroidal vasculopathy examined with en face optical coherence tomography. Clin Experiment Ophthalmol 2007;35(7):596-601.
8. Gomi F, Sawa M, Wakabayashi T, Sasamoto Y, Suzuki M, Tsujikawa M. Efficacy of intravitreal bevacizumab combined with photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 2010 Jul;150(1):48-54.e1.
9. Tomita K, Tsujikawa A, Yamashiro K, Ooto S, et al. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy combined with intravitreal injections of ranibizumab. Am J Ophthalmol 2012 Jan;153(1):68-80.e1. doi: 10.1016/j.ajo.2011.07.001. Epub 2011 Sep 9.
10. Sakai T, Ohkuma Y, Kohno H, Hayashi T, Watanabe A, Tsuneoka H. Three-year visual outcome of photodynamic therapy plus intravitreal bevacizumab with or without subtenon triamcinolone acetonide injections for polypoidal choroidal vasculopathy. Br J Ophthalmol 2014 Jul 22. pii: bjophthalmol 2014-305189. doi: 10.1136/bjophthalmol-2014-305189.
11. Bressler NM, Bressler SB, Hawkins BS, et al. Submacular surgery trials randomized pilot trial of laser photocoagulation versus surgery for recurrent choroidal neovascularization secondary to age-related macular degeneration: I. Ophthalmic outcomes submacular surgery trials pilot study report number 1. Am J Ophthalmol 2000;130:387-407.
12. Chang W, Garg SJ, Maturi R, et al. Management of thick submacular hemorrhage with subretinal tissue plasminogen activator and pneumatic displacement for age-related macular degeneration. Am J Ophthalmol. 2014;157:1250-7.
13. Kung YH, Wu TT, Hong MC, Sheu SJ. Intravitreal tissue plasminogen activator and pneumatic displacement of submacular hemorrhage. J Ocul Pharmacol Ther 2010;26(5):469-74.
14. Shiraga F, Matsuo T, Yokoe S, et al. Surgical treatment of submacular hemorrhage associated with idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol 1999;128:147-54.
15. Sato T, Kishi S, Watanabe G, et al. Tomographic features of branching vascular networks in polypoidal choroidal vasculopathy. Retina. 2007;27:589-594.