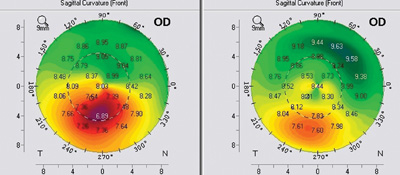
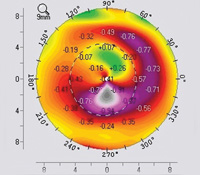
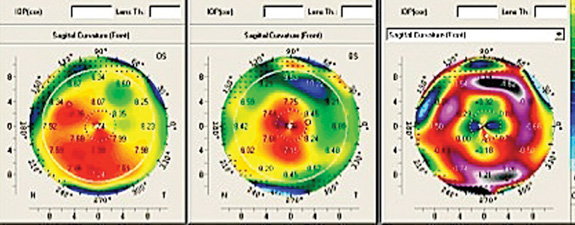
Laser ablation is a complex operation; it depends on precision laser and blade technology, surgical skill and precise measurement of the eye being corrected. But other issues are equally significant—including the source of the measurements on which you base your ablation. Use of wavefront technology to measure the eye’s optical system has become commonplace, but outside the United States ablations are often based on corneal topography.
The technology used to accomplish topography-based laser ablation isn’t yet approved by the U.S. Food and Drug Administration, but a Phase III trial is currently under way. Early results have been reported to be good, so this technology will likely be available in the United States within the next few years. Nevertheless, most American surgeons have little acquaintance with topography-guided ablation.
With that in mind, three surgeons who have used it extensively offer their insights into its pros and cons, and the circumstances in which it may be a better approach than standard or wavefront-guided ablation.
Addressing Corneal Issues
The hallmark of topography-guided ablation is that it’s designed to address corneal issues exclusively, with an emphasis on smoothing or normalizing the anterior corneal surface. As a result, it’s often used to treat corneal abnormalities such as scars or keratoconus. But because it relies on topography for guidance, it arguably accomplishes some things—such as centering the treatment on the line of vision—better than pupil-oriented measuring technologies such as wavefront, even in normal eyes. In addition, it sometimes uses ablation schemes that are significantly different than a wavefront-guided ablation would use to achieve a given result, with potentially significant consequences for the cornea and the eye.
|
Surgeons outside the United States who have used the technology for many years have found that it can benefit less abnormal eyes as well. Aleksandar Stojanovic, MD, who is in charge of refractive surgery at the University Hospital in North Norway and medical director at SynsLaser Clinic in Oslo, Norway, has used topography-guided lasers since 2002. “Norway is a small country, and our clinic gets most of the cases of irregular astigmatism that require laser treatment,” he says. “This has become my niche.
“At the outset, we treated mostly decentered LASIK cases,” he continues. “But over time, our use of this technology has broadened. Now I use it on most virgin eyes as well. The purpose in these cases is not so much to get rid of higher-order aberrations, because in virgin eyes there are not many to remove anyway. Instead, I use the data acquired by topography so the ablation will be perfectly centered and the cornea will be reshaped in a more physiologic manner.”
Topo-guided: The Advantages
A. John Kanellopoulos, MD, clinical professor of ophthalmology at NYU Medical School and medical director of the Laservision.gr Institute in Athens, Greece, says he has worked with topography-guided laser treatments, using the Wavelight/Alcon platform, for the past 10 years (outside the United States).
“This technology has been approved in the European Union since early 2003,” he notes. “For years, we’ve observed the advantages of topography-guided treatments and how they can increase the outcome efficacy and safety of laser treatments to the cornea.”
| ||||
Advantages of this technology—beyond treating very abnormal corneas—include:
• It may be better for treating astigmatism. “When we treat astigmatism in the standard way we treat a given amount of astigmatism as if it’s exactly the same on every eye,” Dr. Kanellopoulos points out. “Clinical experience and topography have proven that there’s a great variation in the way astigmatism occurs. You can have 2 D of astigmatism with a thicker bowl, a thinner bowl or a slightly decentered bowl. Doing topography-guided treatment addresses these differences far more elegantly than the standard one-size-fits-all approach.”
• Surgeons are familiar with topography maps. “It’s much easier to make sense of a topographic map than a wavefront map, which is a very theoretical model,” notes Dr. Kanellopoulos. “Any corneal surgeon will be comfortable basing a treatment on a topographic evaluation.”
• It may create smoother transition zones. In certain topography-guided laser systems, according to Dr. Stojanovic, transition zones may be smoother when correcting corneal higher-order aberrations. The ablation-planning software creates a customized connection between the treated and untreated cornea. “I have an option in my topography-guided laser system to choose whether to just correct lower-order aberrations and customize only corneal asphericity and centration, or to also correct the corneal higher-order aberrations,” he explains. “I’ve experimented with this over the years. For example, I tried correcting only the lower-order aberrations, and then correcting the higher-order aberrations to see what the difference would be. The only big difference I found, in treating virgin eyes, was that my transition zones are better if I correct the higher-order aberrations. Apparently this addresses the fine features in the transition area.
“It’s especially important to me to create very smooth and wide transition zones, because I do most of my cases with surface ablation, not LASIK,” he continues. “That helps avoid some epithelial remodeling issues. This may be less of a concern in LASIK ablations, because the flap helps smooth the transition zones, just like putting an object under a rug. Of course, when we remove the epithelium and it grows back, it also smoothes out the transition zones somewhat, but if the transition isn’t smooth enough, regression will easily occur.
“The issue of epithelial smoothing is also one of the arguments against treating other than first-surface higher-order aberrations registered by wavefront,” he adds. “Imprinting some fine, detailed features on the corneal stroma is really not going to work because of the smoothing during re-epithelialization, or due to the smoothing effect of the flap. So, higher-order aberrations must really be of some significance to be worth attempting to treat. Of course, that is the case in irregular astigmatism.”
• Direct data input. “Topography-guided treatment involves a direct relationship between diagnostics and data processing on the laser,” Dr. Kanellopoulos points out. “It’s a continuous chain. That eliminates the potential error of treating with the wrong patient’s data. If you import topography data from a patient, you know it’s the right program for the patient. It’s a nice safety feature to have in a busy clinical environment.”
• It addresses the issue of angle kappa. “One inherent advantage of topography treatments is that since they’re based on the topographic image of the cornea, whether produced by Scheimpflug technology or placido disc, by definition the image is centered on the apex of the cornea,” says Dr. Kanellopoulos. “The line of sight, or visual axis, is closer to the apex of the cornea than the pupillary center. That means that these treatments are more centered on the visual axis of the patient than other treatments that center the ablation based on the pupil.”
|
Dr. Kanellopoulos adds that some myopes have angle kappa as well. “We’re in an era in which we’re striving more and more for perfect outcomes,” he says. “That includes centering on the line of sight or visual axis or corneal apex, all of which can be deviant to the center of the pupil. If we agree that treatments should be centered on one of these landmarks, then we should evaluate the distance between the center pupil and that landmark in every patient. To accomplish that, you can use diagnostic placido disc imaging of the cornea, which makes it very easy to ascertain whether there’s angle kappa. If there’s angle kappa, the pupillary center won’t match the center of the placido discs.
“We use this diagnostic on every patient,” he says. “I believe it’s a must to use topography-guided treatment in normal hyperopic corneas. In normal myopic corneas, it’s something to consider. Probably only one out of 10 or 20 myopic eyes will have measurable angle kappa, but I still think that’s a significant group—five percent of myopic patients being more than 100 µm decentered is worth addressing.
“Beyond that,” he adds, “we use topography-guided ablation in 20 to 30 percent of myopic astigmats.”
Addressing Spherical Error
One factor that some surgeons see as a disadvantage of topography-guided treatment is that it may not always produce the desired spherical refractive power outcome. “The topographic data are derived from the cornea,” notes Dr. Kanellopoulos, “so if the cornea is highly irregular, the main point of the treatment is to normalize the cornea. As a result, you may end up with a very normal cornea—but with added or reduced sphere. So, in an eye that started with best corrected visual acuity of 20/50, your end result may be a BCVA of 20/20, but now with 2 D of myopia or hyperopia. That’s because the actual refractive power of the whole eye is difficult to calculate when you normalize the cornea. You know you’re going to get a better sphere on that cornea, but you don’t know exactly what spherical power the revised cornea will be.
“There’s a little art involved in determining the likely spherical shift, and there are several techniques that can be used,” he continues. “For instance, if you’re enlarging a small optical zone in a myope, you know that the treatment is going to be a hyperopic-like ablation, so you can predict what the spherical shift in that eye will be. For instance, if your hyperopic-like ablation is at a depth of 30 µm, you know that this eye will shift about 1.5 D toward myopia. That’s an easy prediction.
“On the other hand, suppose you have an eye that’s plano after LASIK and you want to enlarge the optical zone because the patient has night halos causing problems with driving,” he says. “If you try to enlarge that optical zone, most topography-guided treatment plans will create a ring at the edge of the older myopic treatment zone. If you don’t take into account the potential shift in corneal curvature, the eye may end up becoming -1.5 D. The patient won’t have halos at night, but you’ve induced 1.5 D of myopia. In that situation, a second purely spherical treatment may be required.
“However, in most simple interventions such as optical zone recentrations or enlargements, you can predict the spherical change that normalization of the cornea will induce with a high level of certainty,” he says. “In that situation, all you have to do is add it into the treatment. A number of surgeons work in this way, and the results in this type of situation are highly predictable. But if you’re dealing with an extremely irregular cornea, such as one that has a paracentral scar, then all bets are off, because now you’ll have a very complex laser treatment that’s part hyperopic and part myopic. That makes it very difficult to predict which way the overall spherical refraction is going to go. In that situation, a second treatment may be required.”
Dr. Stojanovic acknowledges that topography-guided treatment may not fully address the spherical component of the refraction. “I say to these patients, ‘OK, the first step is to get your quality of vision right, to get rid of your higher-order aberrations—your double vision, haloes, scattering and so forth,’ ” he explains. “As much as we can, we try to provide sphero-cylindrical correction as well, but with the majority of these patients, the lower-order aberrations are not the big problem. These people will gladly use glasses again if they only can get rid of their higher-order aberrations. The sphero-cylindrical correction is of secondary importance to these patients.”
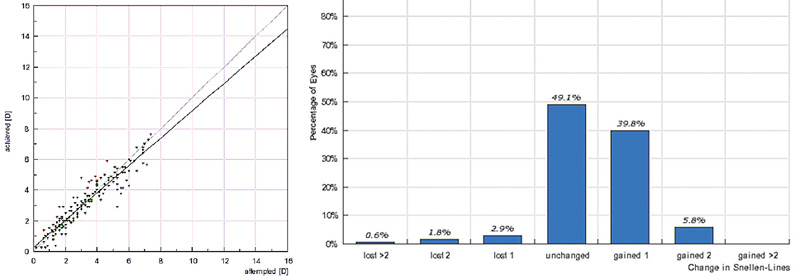 |
| Left: Predictability of topography-guided spherical error correction in 177 hyperopic eyes of 86 patients (age: 42.5 ±11.31 years) at 12-month follow-up. Right: Change in distance visual acuity at the 12-month visit. |
Placido vs. Scheimpflug
Once a surgeon decides that topography-guided ablation is the right procedure for a given patient, another decision must be made: Which topographic source should provide the data?
Dr. Kanellopoulos notes that most doctors are well-acquainted with the two basic types of topography: placido disc and tomography. “Placido disc devices analyze the projection of mires onto the cornea, while the tomographic devices analyze a Scheimpflug or slit-lamp image and derive an elevation map,” he explains. “In either case, the elevation data are input into the laser, and the laser software uses a best-fit sphere concept to normalize the cornea. In fact, we evaluate the use of both types of topography when doing topography-guided ablations because there are advantages and disadvantages to each of them.
| ||
|
Dr. Kanellopoulos notes that in the majority of cases, the images and treatment plans derived from the two different types of devices are identical. “I have access to four different topographers in our clinic; two placido disc peripheral devices and two Pentacam devices, one standard and one high-resolution,” he says. “All of them can feed into our treatment platform. You have to use clinical judgment and consider clinical images of the cornea to decide which of the technologies you should use.
“Over time your experience tells you which one will be more helpful for the treatment you’re trying to make, but I’d say 90 percent of our topography-guided treatments are done using the Scheimpflug technology,” he says. “However, if there’s any issue with cornea translucency or any issue involving peripheral irregularity, we use placido disc. As noted, Scheimpflug is more accurate in the central five millimeters of the cornea, and in my opinion, placido is more accurate in the area between three and six millimeters from the corneal center.” (Dr. Kanellopoulos points out that the current FDA trial is being done using placido-based images.)
“Scheimpflug-based technology gives us solid, primary corneal elevation data, but its curvature measurements are not as detailed as placido’s,” notes Dr. Stojanovic. “However, there are limits to our laser systems, and limits to how much detail can be printed on the cornea without being lost to remodeling. So there’s no point to using extremely detailed information from the placido-based system when we know that this won’t be translated into ablation.
“In addition,” he continues, “we know that placido measurements are prone to so-called ‘cumulative error’ inherent in recalculation from curvature-diopters to microns of elevation—the ‘language’ our lasers understand. For these reasons, I think the Scheimpflug system is a more robust source of topographic information—provided that you’ve done a well-balanced, thorough examination and taken all the relevant factors into account.”
Topography vs. Wavefront
Both wavefront and topography-based ablation have advantages and disadvantages under different circumstances:
• Different ablation strategies. When comparing outcomes using wavefront-guided and topography-guided ablation, Dr. Kanellopoulos notes that the two technologies use very different ablation strategies to achieve the desired refraction, especially when treating an irregular cornea. “There’s an intrinsic advantage in trying to do this with topography instead of wavefront,” he says. “With topography, if you’re looking at peaks and valleys, you don’t necessarily have to bring all of the peaks to the valley level. You can buff off the tops of the peaks and then treat adjacent to the valleys, thus making the valleys steeper. Using that approach, the amount of tissue removal required to normalize an irregular cornea is much smaller than would be required with wavefront.
“Suppose you have a bump on the cornea,” he continues. “Think of it as being like a 12-story building. Wavefront technology assumes you have to bring the 12th floor down to the ground floor, so you have to remove all of that tissue. With topography you can bring the 12th floor down to the eighth floor level and then use steepening to do the equivalent of bringing the ground level up to sixth-floor level. That significantly reduces the amount of tissue removal. It’s similar to the way hyperopic treatment works. In order to steepen the cornea we ablate a ring of tissue in the midperiphery. Even though we haven’t touched the cornea in the center, the center becomes steeper.
“For this reason, when a very irregular cornea is normalized using a topography-guided ablation, the treatment is a combination of small myopic and hyperopic ablations working in synchrony to create a more normal surface—with less tissue removal from the peaks. This is very important, because corneal peaks are usually associated with thinner corneas. Of course, that’s not always the case; for instance, with a contact-lens-related scar, the scar area is flatter and the cornea next to it is steeper. But in idiopathic irregularities such as keratoconus, the steeper cornea is usually the thinner part of the cornea. In those situations it becomes a significant issue to try to avoid removing too much tissue from the steeper areas.”
Along these lines, Dr. Stojanovic points out that pairing topography-guided ablation with cross-linking to treat keratoconus patients works well because it may create a clear optical center without ablating a large amount of corneal tissue. “The patient gets a good central window with fewer higher-order aberrations at the same time as we do cross-linking to stabilize the keratoconus,” he explains. “We’ve done this procedure many times, and have had very good results, similar to those of Dr. Kanellopoulos.”1
• Reproducibility of measurements. Another difference between wavefront-based ablation and topography-guided ablation, according to Dr. Kanellopoulos, is the stability of the measurements. “Topography is a far more stable parameter to evaluate than wavefront, because it has far less fluctuation,” he says. “Wavefront is a dynamic measurement, so there is no perfect wavefront. Of course, there’s no perfect topography either, but if you compare serial topographies of the same patient from a Pentacam or high-end placido disc device, the standard deviation of fluctuation is miniscule compared to the fluctuation of the parameters on wavefront maps.
“Wavefront maps,” he continues, “are very much affected by factors such as centroid shift; the state of the pupil you’re measuring; the eye’s accommodative state; whether you’re measuring monocularly or binocularly; and so forth. The literature supports the idea that there isn’t a single, steady, reproducible wavefront measurement. We try to make them consistent by dilating the eye and using dark rooms, but this is not a physiologic measurement. Nobody puts a dilating drop in before he gets in his car to drive at night, so why would this be an ideal wavefront for that person?
“The information you get from wavefront technology includes the crystalline lens, which is dynamic,” agrees Dr. Stojanovic. “It’s kind of a flying target; the readings will be different at distant and close focus, and likely so from measurement to measurement. Besides, I would never want to correct lenticular problems at the corneal level, and that’s what you’re doing in many cases if you rely on wavefront blindly. You’re basing your treatment on one moment in time when the wavefront aberrometry was taken, and it may not be representative of the optics of the eye.
“Ultimately, I believe custom ablation, in the sense of treatment of higher-order aberrations, is really necessary for treatment of irregular corneas,” he says. “And if you’re going to treat the cornea, you should measure the cornea, not the wavefront of the whole eye.”
“Our experience has been that if you have both modalities available and you’re efficient at both, you’ll probably do 100 topography-guided treatments for every wavefront-guided treatment,” adds Dr. Kanellopoulos. “Wavefront technology is a good tool, but there are a lot of variables that make it less reproducible.”
• Differences in what you’re correcting. Dr. Kanellopoulos points out that most topographers can produce wavefront imaging based on corneal irregularities. “More than 90 percent of wavefront irregularities are corneal irregularities,” he says. “So if you’re talking about the majority of people with regular corneas and how you determine which ones need treatment, a topographic map does a great job of pointing out irregularities. It’s a very easy map to read.
“Suppose a patient has coma on the wavefront map generated by the topographer,” he continues. “How do you correct that coma? You can do a wavefront-guided ablation. But suppose you have smaller tissue reserves, or you don’t want to manipulate the rest of the wavefront indices that will be implicated in coma. In that case, you can do a topography-guided ablation. That will just correct the coma, Zernike C-6 and C-7, and potentially improve the asphericity, C-12. By definition, topography-guided treatments have the ability to improve corneal asphericity. So now you have a ‘wavefront-guided treatment’ that manipulates C-6, C-7 and C-12 only. And these are probably the most reproducible indices in wavefront and in topography.”
|
Dr. Kanellopoulos says that he sees improving a limited number of Zernike factors as the biggest advantage of doing topography-guided ablation rather than wavefront-guided. “Topography-guided ablations improve spherical aberration,” he says. “That’s why their results are superior to standard treatments. And that’s why the wavefront-optimized outcomes data submitted to the FDA were at least equal to the wavefront outcomes data. People say you should do wavefront-guided treatments because you improve C-12, but other platforms accomplish the same thing.”
Dr. Stojanovic agrees. “I think the main thing wavefront is taking credit for is optimizing the ablation to avoid inducing spherical aberration,” he says. “The companies introduced wavefront together with aspheric treatments, which address the issue of corneal asphericity. They credited wavefront with this fantastic achievement. I think most of the improvement ascribed to the use of wavefront technology was the result of two things: optimizing the asphericity by creating the right ablation profile, and centering the ablation on the optics of the eye instead of the center of the pupil. These were great improvements over the standard ablation that came before it, but I believe the wrong technology took credit for them.”
Dr. Kanellopoulos notes another concern: correcting aberrations that are helpful. “Wavefront-guided ablation changes other indices that affect vision in ways we don’t fully understand,” he says. “Some wavefront aberrations, for example, help us to see by enhancing our ability to make out details. We’ve seen proof of this in studies performed on fighter pilots. The majority of fighter pilots have transverse coma between the two eyes, with the dominant eye vertical and the nondominant eye horizontal. Do we really want to ‘correct’ that?”
“Topography-guided custom ablation treatment, or T-CAT, in general has several potential advantages over wavefront-guided treatment, particularly in the treatment of people who have aberrated corneas from previous refractive surgery,” summarizes Dr. Stulting. “First, the measurements are more accurate than wavefront measurements. Second, the measurements are not pupil-dependent; topography gives us more accurate information about the periphery of the cornea, which is where most of the aberrations lie. Third, topography isn’t affected by internal optical components such as early cataract.”
However, Dr. Stulting notes that topography-guided ablation has a few downsides. “T-CAT won’t allow us to correct for any intraocular aberrations, if desirable, and topographic measurements don’t give us any information about optical correction,” he says. “So, you have to correct the corneal aberrations and then correct the optical error of the eye using two separate and independent measurements. And, it’s a lot more work to do it correctly than it is to do conventional or wavefront treatments. It’s more painstaking to make sure that the data that generate the treatment algorithm don’t contain any artifacts.”
When is Topo-guided Best?
Dr. Kanellopoulos admits that the majority of patients don’t require topography-guided treatment. “I don’t want to come across as saying that most cases need this,” he says. “However, hyperopes, which represent 10 to 14 percent of the population being treated, are a significant subgroup. Myopes with significant astigmatism could be added to that group. And of course, it’s a good option in challenging cases, such as complications, corneal irregularities, corneal scars, decentered ablations, or trying to fix older technology mishaps and imperfections. However, dealing with patients in these challenging cases requires a certain amount of acquired skill.”
Dr. Stojanovic says he would not use this technology to treat higher-order aberrations if the data suggests that corneal and lenticular aberrations are cancelling each other. “Because that is a possibility, we measure both crystalline lens aberrations and corneal aberrations,” he says. “In cases where you find more corneal higher-order aberrations than total system aberrations, topography-guided custom ablation should be used. If some of the lenticular aberrations become manifest after such treatment, I believe those will diminish greatly over time, since the crystalline lens is a dynamic structure and can adjust itself to compensate for corneal higher-order aberrations to a certain degree.
“An OPD scan, and a couple of other systems, measure higher-order aberrations in both locations,” he adds. “If I find a balance between the corneal and internal aberrations, then I leave the corneal higher-order aberrations alone. That’s especially true if the patient is younger and pre-cataract—which most laser-correction patients are. If the patient is older and likely to get rid of his cataract in the near future, I’m more likely to proceed with regularizing the cornea.”
Pearls for Beginners
When topography-guided ablation finally does become available in the United States, there are a few pitfalls surgeons can avoid at the outset:
• Be careful about the topography. “The most important advice I can offer new users is to be certain of the quality of the topography,” says Dr. Stojanovic. “Unfortunately, this can be difficult to achieve when there is irregular astigmatism; the topography is difficult to measure and prone to artifacts. So, spend as much time as necessary to be sure you have good topographic information before you start basing your ablation on it. Don’t just get the topography from the technician and put it into the software. That’s a mistake early users often make. You have to think more carefully and question the data. Does it make sense with the patient’s symptoms? Will this treatment address the problem we really want to treat?”
• Don’t attempt this on complex corneas until you’ve become more experienced with it. “If you’re going to make radical changes on the cornea—which is required in a very irregular cornea, a scar, a very eccentric previous ablation or a keratoconic eye—then you have to be concerned about the amount of sphere and cylinder you’re adding to the treatment,” says Dr. Kanellopoulos. “The spherical change that an irregular treatment induces is only partially predictable, so you probably shouldn’t jump into using topography-guided ablation with these types of cases at the outset.”
• Take the tear film into account. “An uneven or broken tear film will certainly introduce artifacts into your topography measurements,” says Dr. Stojanovic. “If you base your treatment on artifacts you’re in trouble. To avoid that, pretreat any dry eye and improve the tear film.”
Coming Soon?
Dr. Stojanovic notes that, unfortunately, innovation and development in topography-guided laser ablation have been very limited. “Topography-guided laser ablation has been a niche market for many years,” he observes. “The leading manufacturers haven’t invested a lot of R&D money into improving the technology because there’s not much money in it. Not a lot of patients are currently being treated with it.
“In a way, this may be a self-perpetuating cycle,” he continues. “The software behind this technology hasn’t been refined very much, so the surgeon has to think carefully about what he or she is doing and make adjustments because the software doesn’t do everything for you. As a result, many doctors shy away from treating irregular astigmatism with topography-guided custom ablation—thus keeping the market small. Hopefully, now that Alcon has entered the market, they’ll refine the software for this application.”
Despite its limitations, Dr. Kanellopoulos says topography-guided ablation is an extraordinary tool. “There’s no doubt in my mind that using topography-guided treatment, either through the placido disc pathway or Scheimpflug image pathway, has produced excellent results, especially in the small percentage of irregular eyes that we deal with every day in our refractive surgery practice,” he says. “This had been a dead end for these patients for many years—but it isn’t any longer.”
“I think many American surgeons are not aware of the theoretical advantages of topography-guided treatment,” adds Dr. Stulting. “However, those who are familiar with the methodology and outcomes are anxious to have it. This technology certainly should be part of our armamentarium.” REVIEW
1. Stojanovic A, Zhang J, Chen X, Nitter TA, Chen S, Wang Q. Topography-guided transepithelial surface ablation followed by corneal collagen cross-linking performed in a single combined procedure for the treatment of keratoconus and pellucid marginal degeneration. J Refract Surg 2010;26:2:145-52.