 |
Refraction provided no improvement in vision. There was no evidence of surface dryness or irregularity. A retina consultation was obtained with a complete dilated fundus exam, fundus photos and an OCT. The vision at this time had decreased to 20/60 in the right eye and 20/30 in the left eye, with an IOP of 6 mmHg OU. The overall impression was that there was no acute retinal process. Dry age-related macular degeneration with minimal macular wrinkling was present in the right eye. This was thought to be visually insignificant. Mild non-proliferative diabetic retinopathy was noted in both eyes, but no clinically significant macular edema was observed.
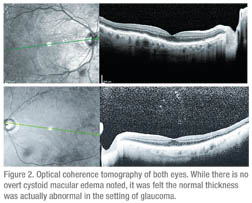
A referral to neuro-ophthalmology was subsequently made for further evaluation. Vision in the right eye was 20/70 and 20/40 in the left eye with IOP measuring 2 to 4 mmHg in the right eye and 14 mmHg in the left. Extraocular motility, confrontational visual fields and pupils were normal. Color vision was full in the right eye and 5/10 in the left. Fundus exam showed stable dry AMD and NPDR. After reviewing the OCT (See Figure 2), it was felt the visual decline was secondary to hypotony maculopathy.
Discussion
In this case, a cyclodialysis cleft was intentionally created during cataract extraction for the treatment of progressive glaucoma. A cyclodialysis cleft is a disinsertion of the longitudinal fibers of the ciliary muscle from the scleral spur. This creates a communication between the anterior chamber and the suprachoroidal space resulting in a decrease in intraocular pressure. Although now rarely utilized, this reduction in IOP was desirable, and surgical formation of a cyclodialysis cleft was once a common surgical procedure used to treat glaucoma. Cyclodialysis clefts can also be unintentionally created during blunt or penetrating trauma and during intraocular surgery. Complications secondary to the unusually low IOPs created in the setting of a cleft include shallow anterior chamber, induced hyperopia, cataract, choroidal effusion, retinochoroidal folds and optic disc edema.
Evaluations of patients with a cyclodialysis cleft can be difficult because the eye is soft, and there may be associated injuries such as sphincter tears or hyphema. Some helpful techniques to deal with these issues include intracameral viscoelastic with or without pilocarpine, anterior segment OCT (as there is no contact), ultrasound biomicroscopy and scleral transillumination.
Medical management includes 1% atropine twice daily, which relaxes the ciliary body, allowing apposition. Alternatively, apposition can be achieved by reducing postop steroids, which encourages inflammation. Argon laser photocoagulation, transscleral diode photocoagulation and transscleral YAG laser cyclophotocoagulation can occasionally be successful in closing the cyclodialysis cleft. Transscleral diathermy using a partial thickness scleral flap or transconjuctival cryotherapy with or without a gas bubble has also been suggested. Suturing the ciliary body to the sclera or “cyclopexy” is the usual procedure of choice.
Michael Küchle, MD, and Gottfried Naumann, MD, reported on 29 consecutive patients with traumatic cyclodialysis clefts treated with direct cyclopexy, with all but one achieving complete closure and the remaining patient having no hypotony. A variable period of high postoperative IOP is to be expected. Some newer surgical techniques include anterior buckling with a silicone rod under a partial scleral flap (with or without cryotherapy and anterior sponges above the cleft), a capsular tension ring or large IOL haptics for internal cerclage, and gas endotamponade in conjunction with cryotherapy and pars plana vitrectomy. It is not clear that these offer any advantage over direct suture closure of the cleft.
The cause of vision change in this patient was presumed to be hypotony maculopathy secondary to the low intraocular pressure as a result of the cyclodialysis cleft. Hypotony itself is defined in two ways; statistical hypotony is IOP <6.5 mmHg, or three standard deviations below normal. Clinical hypotony is low IOP resulting in visual loss in the form of keratopathy, cataract, choroidal effusion or astigmatism. In addition, hypotony causes fundus abnormalities including disc edema, vascular tortuosity and chorioretinal folds/wrinkling as the scleral wall collapses. Causes of hypotony maculopathy commonly include glaucoma procedures or trauma resulting in low IOP (perforating injuries, cyclodialysis cleft, etc.). Hypotony maculopathy is more likely to occur in patients with young age, myopia, primary filtering surgery or systemic illness. The cystoid macular edema in hypotony maculopathy is thought to result from abnormal retinal capillary permeability secondary to a reduction in interstitial pressure, while the disc edema is likely secondary to anterior bowing of lamina cribrosa, which constricts axonal bundles and reduces axoplasmic transport.
The diagnosis of hypotony maculopathy can be made with the help of imaging. OCT is best for subtle macular fluid or folds that may be missed on fundoscopic exam. Fluorescein angiography is helpful, as pure retinal folds do not alter background fluorescence as seen with scleral folds. B-scan ultrasonography can be helpful when posterior view is poor, as well as to exclude other pathology. The appropriate treatment depends on the cause of hypotony.
In this case, the patient chose not to have surgical treatment. His right eye had reduced acuity due to the hypotony maculopathy but his visual field was full. His left eye had good central acuity but a markedly constricted visual field due to advanced glaucoma. While the surgery would likely improve his central vision in his right eye, it would ultimately not cause any improvement in his function or quality of life.
The author would like to thank Robert Sergott, MD, Wills Eye Institute Neuro-Ophthalmology Department, and George Spaeth, MD, Wills Eye Institute Glaucoma Department, for their time and assistance.
1. Azvara-Blanco A, Costa VP, Wilson RP. Handbook of Glaucoma (eds.), 2002, London: Martin Dunitz.
2. Costa VP, Arcieri ES. Hypotony Maculopathy. Acta Ophthalmol Scand 2007:85:586-597.
3. Ioannidis AS, Barton K. Cyclodialylsis Cleft: Causes and repair. Curr Opin Ophthalmol 2010 21:150-154.
4. Küchle M, Naumann GO. Direct cyclopexy for traumatic cyclodialysis with persisting hypotony. Report in 29 consecutive patients. Ophthalmology 1995;102:322-333.
5. Omerod LD, Baerveldt G, Sunalp MA, Riekhof FT. Management of the hypotonous cyclodialysis cleft. Ophthalmology 1991;98:1384-1393.



