Bacteria are made to adapt. It’s been known for some time that some current classes of antimicrobial drugs are becoming less effective against some common bacteria.
The Infectious Disease Society of America recently issued a policy paper on combating antimicrobial resistance as part of its Special Area of Focus titled “Bad Bugs, No Drugs … 10 New Antibiotics by 2020.” According to this paper, “the issue has reached a critical point, as bacteria are becoming increasingly resistant to the antibiotics we have, and new drugs are not being developed at anywhere near the pace necessary.”1
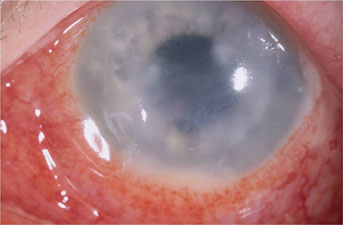 |
| Methicillin-resistant Staphylococcus aureus keratitis post-LASIK. |
Another concern is multidrug-resistant Staphylococcus epidermidis. A recent study conducted by Stephen J. Kim, MD, and colleagues at Vanderbilt University analyzed the emergence of the strain after repeated conjunctival exposure to topical macrolide or fluoroquinolone antibiotics.2 In this study, 48 eyes of 24 patients received four consecutive monthly unilateral intravitreal injections. Each patient was randomly assigned to one of four antibiotics: azithromycin 1%; gatifloxacin 0.3%; moxifloxacin 0.5%; or ofloxacin 0.3%.
After four consecutive treatments, 58 isolates of S. epidermidis were isolated from control and treated eyes. Resistance to three or more antibiotics was observed in 69 percent of S. epidermidis isolated from control eyes and 90 percent of S. epidermidis isolated from treated eyes. Additionally, a total of 46 S. epidermidis isolates from control eyes and 38 S. epidermidis isolates from treated eyes were cultured from the fifth treatment until the final injection. Resistance to five or more antibiotics was present in 48 percent of control eyes compared with 71 percent of treated eyes. S. epidermidis developed resistance to third-generation and fourth-generation fluoroquinolones in significantly more fluoroquinolone-treated eyes compared with control eyes.
These bacteria also developed resistance to trimethoprim/sulfamethoxazole, gentamicin, and clindamycin. And, significantly more azithromycin-treated eyes developed S. epidermidis that were resistant to macrolides, trimethoprim/sulfamethoxazole and doxycycline compared with control eyes.
In a separate study, Dr. Kim found substantial levels of resistance to third- and fourth-generation fluoroquinolones and multiresistance among ocular coagulase-negative staphylococci isolated from patients undergoing intravitreal injections for choroidal neovascularization.3
How We Got Here
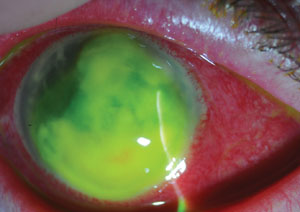 |
| Corneal ulcer due to Staphylococcus aureus. |
According to Dr. Gilmore, one problem is that it is more economical to prescribe a broad-spectrum antibiotic and not worry about which bacterium is the cause. “That’s because the treatments are relatively cheap compared to the tests for determining the cause. The problem is that the more bacteria that experience the drugs, the more bacteria that are going to spend time figuring out how to get around it,” he says.
Determining the cause of infection and focusing treatment could extend the lifetime of current drugs; however, diagnostics are expensive. “But, I think that’s where the future is going,” says Dr. Gilmore. “We’re never just going to outrun these by brute force, and that’s what we’ve been trying to do. We’ve just been trying to come up with a cannon to treat the problem. It’s sort of like clearing the forest to get rid of some weeds that we’re worrying about. We need to go after just the weeds. Once we do that, our drugs will have much longer shelf lives.”
Dr. Bertino agrees, noting that antibiotics have been used and continue to be used in food-chain animals, which contributes to resistance. “In the late 1970s, we knew that antibiotic use in chickens spawned development of resistant bacteria in human beings,” he says. “If you are a chicken farmer and you have 100,000 chickens in one building and 5 percent of them are sick, you can’t treat 5 percent of them, so you treat them all with an antibiotic. It’s very clear that antibiotic use in animals has spawned bacterial resistance.”
Antibiotics are also overused because of the public’s lack of knowledge about them. Dr. Bertino notes that many people still take antibiotics for the common cold. Additionally, in many countries, people can purchase antibiotics without a prescription. “You’d be shocked at the number of developed countries where you can walk into a pharmacy and get antibiotics without a prescription,” he adds.
There are agents, such as disinfectants, to which bacteria are unlikely to be able to develop resistance. One example is povidone iodine, which is effective against most pathogens. “In terms of the mechanism of action, bacteria probably will never become resistant to them, but never say never when you have bacteria that can mutate,” says Francis Mah, MD, an associate professor of ophthalmology at the University of Pittsburgh School of Medicine. “But, the issue is toxicity. If it kills indiscriminately and it can kill pretty much any bacteria or fungus or parasite, most times, it is going to cause some amount of toxicity to human cells, like the epithelial cells around the eyes.”
 |
| Resistant strep keratitis. |
New systemic antibiotics include Tygacil, which is in the same family as the tetracyclines, but has broader activity. Additionally, Televancin has activity against MRSA, and it has been approved for systemic use. “Linezolid has found its way into the ophthalmic literature because it has very good activity against gram-positive bacteria. It is available as an oral as well as an IV agent, and it is relatively stable, which has always been a challenge for ophthalmology,” Dr. Mah says.
According to Dr. Kim, the goal with antibiotics is just to prolong the time until resistance is developed. “During the past 10 to 20 years, we have seen new classes of drugs that attack different parts of the bacteria and are advertised to be more effective, but the field is moving toward the realization that antimicrobial resistance is a major concern because we are producing superbugs,” he says. “There are always going to be antibiotics that are slightly better and more effective for a short period, but then they are used inappropriately.”
Dr. Kim notes that there is very little evidence that these antibiotics prevent infection after intravitreal injection, yet they are commonly used for this purpose. “After each intraocular injection, people were prescribing antibiotics to prevent infection. No matter how good the antibiotic is, if it is used inappropriately, bacteria are going to develop resistance to it. Bacteria are very good at developing resistance. They mutate very quickly. We need to have better guidelines, and we probably need to start limiting and restricting the use of antibiotics because of these concerns,” he adds.
A study was recently conducted at the Bascom Palmer Eye Institute to identify the resistance profiles of conjunctival and nasal bacterial isolates in patients undergoing intravitreal injections.4 All patients received three days of moxifloxacin eye drops after each intravitreal injection as prophylaxis against endophthalmitis. Of 93 patients, 42 had at least one fluoroquinolone-resistant organism in the nose or conjunctiva. Additionally, 12 of 25 patients with no previous injections had at least one resistant organism; 14 of 31 patients with one to four previous injections had at least one resistant organism; eight of 24 patients with five to nine previous injections had at least one resistant organism; and eight of 13 patients with 10 or more previous injections had at least one resistant organism.
The Cost Factor
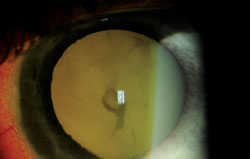 |
| Endophthalmitis due to Cellulosimicrobium cellulans. |
Because many of the large pharmaceutical companies have abandoned researching new antibiotics, Dr. Gilmore recently organized a Harvard-wide project on antibiotic resistance to try to come up with new lead compounds to try to fill the vacuum that’s being created by the loss of big companies’ early discovery programs. “Our hope is to take those leads forward to a point where a company is interested in taking it the rest of the way,” he says. “The discovery process is what they are deciding not to spend much time on. If we don’t keep making discoveries, we can get behind the eight ball fast.”
New Technologies
Because of the obstacles to developing new antibiotics and because they are only effective for a short time, researchers are looking at other ways to keep bacteria in check. Dr. Gilmore points to nanostrings, which are fluorescent “barcodes” that developers say will offer greater disease-detection sensitivity and accuracy than current detection methods. Nanostrings bind to RNA molecules for digital gene expression analysis and have shown promise as an in-office test. “If we could use these to determine the exact problem, then we could treat it directly,” says Dr. Gilmore.
Dr. Mah says that proteomics and genomics also look promising. “This is where you are using the DNA of the bacteria or the protein synthesis or enzymes that the bacteria make themselves to turn cells on or off,” he says. “Bacteria will only grow if there is food, so if there is not enough food, the bacteria will slow down in growth or even stop. If you can trick the bacteria and they are unable to sense the food or if you can turn off their growth, that might be a mechanism. Ten or 20 years from now, we may not be talking about antibiotics. We may be talking about detergents, preservatives and antiseptics, and then using some of these other more innate types of solutions to treat and manage infections.”
The bugs will be waiting.
1. http://www.idsociety.org/uploadedFiles/IDSA/News_Room/Bad_Bugs_Need_Drugs/IDSA%20Policy%20Paper%20Fact%20Sheet.pdf.
2. Dave SB, Toma HS, Kim SJ. Ophthalmic antibiotic use and multidrug-resistant Staphylococcus epidermidis: A controlled, longitudinal study. Ophthalmology 2011 Aug 17. [Epub ahead of print].
3. Kim SJ, Toma HS, Midha NK, Cherney EF, Recchia FM, Doherty TJ. Antibiotic resistance of conjunctiva and nasopharynx evaluation study: A prospective study of patients undergoing intravitreal injections. Ophthalmology 2010;117:2372-2378.
4. Alabiad CR, Miller D, Schiffman JC, Davis JL. Antimicrobial resistance profiles of ocular and nasal flora in patients undergoing intravitreal injections. Am J Ophthalmol 2011 Aug 20. [Epub ahead of print].