How often do glaucoma and neurologic problems overlap? Glaucoma is a common disease, which means patients with brain tumors or other neurologic diseases often have glaucoma as well. In medicine we usually try to explain all of a patient’s symptoms as being caused by a single disease. If someone has headache and numbness, we don’t assume he has two different diseases. But as Hickam’s dictum states: “Patients can have as many diseases as they damn well please.” The reality is that some patients actually do have two problems, and some glaucoma patients also have neurologic disease.
When treating glaucoma, neurologic disease can be easy to miss. In fact, if you treat glaucoma on a regular basis, sooner or later you’re likely to be fooled. That’s true for a couple of reasons. First, the symptoms of many optic neuropathies can mimic those of glaucoma. Intraocular pressure can be normal or elevated in both situations, and visual field defects associated with other optic neuropathies may be identical to those associated with glaucoma.
Furthermore, cupping occurs in many types of optic neuropathy—a fact that’s not well appreciated. In one study, 20 percent of optic atrophy eyes had cupping, and 6 percent of them looked typical for glaucoma.1 Conditions that can cause optic disc cupping include:
• compressive optic neuropathy;
• retrobulbar neuritis;
• papillitis;
• Leber’s hereditary optic neuropathy;
• autosomal dominant optic atrophy;
• infectious optic neuropathy (e.g., syphilis);
• toxic optic neuropathy (e.g., ethambutal);
• trauma;
• central retinal artery occlusion; and,
• arteritic ischemic optic neuropathy.
All of these can occasionally be mistaken for glaucoma and should be considered when you suspect that something other than glaucoma may be going on. It’s worth noting, however, that an MRI isn’t equally helpful in diagnosing everything on this list. Neuroimaging would be most helpful in making a correct diagnosis in compressive optic neuropathy and optic neuritis; other conditions listed above would require different diagnostic approaches.
Here, I’d like to discuss some of the ways you can distinguish between glaucoma and neurologic disease, with an emphasis on which signs and symptoms should make you consider performing neuroimaging of your glaucoma patient.
Warning Signs: Visual Fields
It’s important to take note when visual fields are not typical of glau-coma. Sometimes a patient who has definitely been
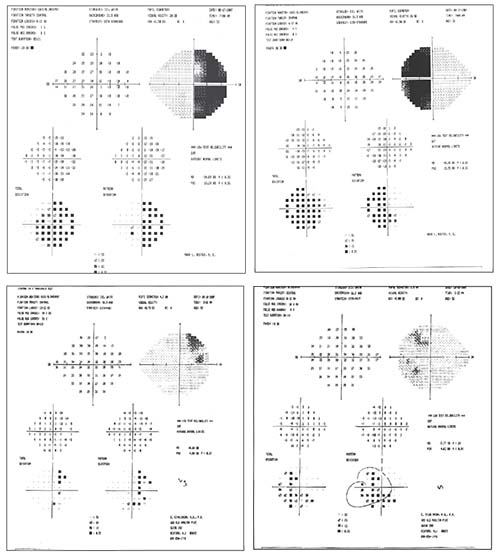 |
| An unusual visual field should alert you to consider a neurological problem. This patient presented with bi-temporal hemianopsia (visual fields, above, left). MRI revealed a large pituitary tumor, compressing and elevating the optic chiasm (left). After surgery, the visual fields improved dramatically (above). |
Although a few visual fields caused by neurologic disease may resemble glaucomatous visual fields, a field that’s distinctly unusual should prompt you to consider a neurologic problem. A typical glaucomatous field might show a superior arcuate defect; but when the visual field reveals a central or cecocentral scotoma; a bitemporal loss; a vertical hemianopic field loss; an inferior altitudinal defect; or visual field loss not proportional to optic disc cupping, you should consider neurologic disease. For example, a visual field that respects the vertical meridian suggests a classic lesion of the optic chiasm. Some defects caused by a neurologic problem may resemble a glaucomatous defect, such as an inferior altitudinal defect. However, you should at least consider the possibility that such a defect could have been caused by an ischemic optic neuropathy—a lack of blood flow to the nerve.
For example, consider the visual fields shown above, from a patient with a classic bi-temporal hemianopsia. Of course, this type of field is atypical for glaucoma, and in fact MRI revealed a large pituitary tumor, compressing and elevating the optic chiasm. It’s worth noting that addressing the cause of the problem via surgery can have a dramatic effect; the visual fields done six weeks postoperatively show a dramatic improvement in the patient’s field.
 |
He came to us for a second opinion. I looked at his optic nerves; they didn’t appear to be significantly cupped. Then I reevaluated his visual fields. The defect in the right eye did appear to be a standard glaucoma defect—it was an arcuate, superonasal defect. But the defect in his left eye was a temporal defect, which is atypical. Furthermore, it bordered on the vertical meridian, which is also atypical. Considering the two fields together, this looked like a left homonymous superior quadrantanopia, suggestive of a lesion behind the optic chiasm on the right.
Given this evidence, we did an MRI; we found a very large meningioma in the brain with adjacent edema. The patient underwent surgery, and as you can see from the visual fields at the bottom, following the surgery he experienced a dramatic improvement in his vision. (In a case like this, no matter which MIGS device you favor, you wouldn’t get a great result with glaucoma surgery!)
Warning Signs: The Optic Nerve
The condition of the optic nerve can provide significant clues to a neurologic problem:
• Remember that cupping can be caused by compressive optic neuropathies. A study conducted at Massachusetts Eye and Ear looked at the cup-to-disc ratio in patients who had
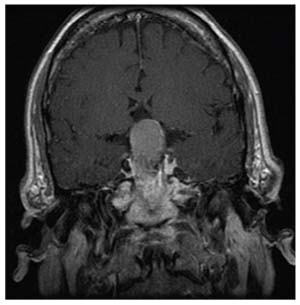
 |
| A 79-year old man being followed for pseudoexfoliation. His IOP remained in the low teens and his optic nerves were not significantly cupped, but he had a superonasal visual field defect in the right eye which progressed over time. MRI revealed a very large meningioma in the brain with adjacent edema. Following surgery, his vision improved dramatically. |
• Note the state of the patient’s visual acuity relative to the cup-to-disc ratio. Optic neuropathies are often associated with poor visual acuity despite a mild increase in cup-to-disc ratio. In contrast, in glaucoma, visual acuity is preserved until cupping is severe.
• Note the timing of cupping. In glaucoma, cupping of the optic nerve is seen before vision loss occurs. In neurologic disease, cupping of the optic nerve is usually seen after visual loss has begun.
• Note the condition of the optic nerve rim. Focal or diffuse obliteration of the rim is relatively specific for glaucoma. In contrast, pallor of the preserved rim is highly suggestive of other causes of optic neuropathy.
Other Considerations
Other strategies that will help you avoid being fooled include:
• Be extra vigilant if the patient is young. Glaucoma tends to be less common in young people, so at least consider neurologic disease in a young person.
• Look for symptoms often associated with neurologic disease. These would include headache, ocular pain and ocular motility defects.
• Note an afferent pupillary defect. A glaucoma patient with a very asymmetric cup or asymmetric field can have a relative afferent pupillary defect (rAPD). But if you don’t find an asymmetric cup or field and you see an rAPD, then you have to think neurologic.
• Note a color vision defect. It’s very important to measure color vision. Although glaucoma causes a dyschromatopsia, it’s usually mild in comparison to dyschromatopsia caused by other optic neuropathies.
• Note any unexpected patterns on OCT scans. OCT is often used when evaluating glaucoma patients. If you see a pattern that doesn’t fit your expectations for a glaucoma patient, consider the possibility of a neurologic problem. For instance, an early binasal loss on a ganglion cell analysis suggests a compressive lesion at the optic chiasm.3
• In general, the more atypical the findings, the more you should consider neurologic disease. Neurologic cases that resemble glaucoma often present with multiple aspects that are not typical of glaucoma. Atypical findings would include an atypical medical history, an atypical exam, an atypical optic disc, a large amount of asymmetry, unexpectedly rapid progression and/or abrupt visual loss. If the history, exam, optic disc and visual fields are all close to what you would expect, you’re probably dealing with glaucoma. But if more than one of those are atypical, your mental alarm should go off: The signs and symptoms you’re seeing could be caused by neurologic disease.
For example, consider the
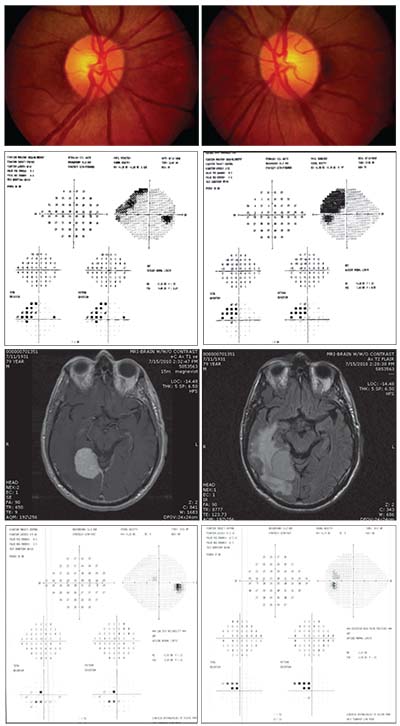
 |
| Focal or diffuse obliteration of the rim is relatively specific for glaucoma, while pallor of the preserved rim is highly suggestive of other causes of optic neuropathy. Above left: Focal partial obliteration of the rim inferiorly in glaucoma. Right: A patient with multiple episodes of optic neuritis. Note cupping, but also pallor of the temporal rim. |
When I met the patient, his IOP was 8 mmHg OD and 10 mmHg OS, but his visual acuity was 20/200 in the right eye—a pretty severe loss for glaucoma. He also had a severe color vision defect in the right eye; he correctly read only one of 13 Ishihara plates; with the left eye he correctly identified all 13. The right eye also had a 3+ afferent pupillary defect. His fundus photos revealed cupping, much greater in the right eye, but also pallor of the right rim. In short, there was a lot that would be considered atypical for a glaucoma patient.
We conducted further testing. A chest X-ray revealed hilar adenopathy; the patient had an elevated angiotensin converting enzyme (ACE) level of 100; and bronchoscopy revealed noncaseating granulomas. It became clear that he was suffering from sarcoidosis with optic neuropathy; he actually didn’t have glaucoma at all. Although we didn’t redo the MRI scan, had the first scan been focused on the orbit, it most likely would have revealed that the optic nerve was inflamed by the sarcoidosis.
Performing Neuroimaging
What kind of tests should you do for neuroimaging? We prefer an MRI of the brain and orbit. A CT scan is a good second choice if an MRI is contraindicated. In either case, it’s crucial to communicate with the radiologist to make sure you’re getting the proper scan of the proper area. You don’t want to end up with a brain MRI without contrast that doesn’t show the visual pathway well; you could easily miss the lesion you’re looking for.
There’s been some debate regarding neuroimaging of patients with typical normal-tension glaucoma. One way to address the possibility that some of these patients could have a neurologic problem would be to neuroimage all of them. However, this would be a major expense and inconvenience, and published findings on the value of such a protocol have been mixed. In one study by Ike Ahmed, MD, 62 patients with newly diagnosed typical normal-tension glaucoma underwent neuroimaging; four of them (6.5 per-cent) had compressive lesions (two pituitary tumors, one meningioma and one arachnoid cyst).4 However, other studies have not found any lesions. A 1998 study involving 52 eyes of 29 normal-tension glaucoma patients found no compressive lesions;5 and a 2010 survey of 68 normal-tension glaucoma patients who had undergone CT scans and carotid Doppler scans revealed no other lesions.6
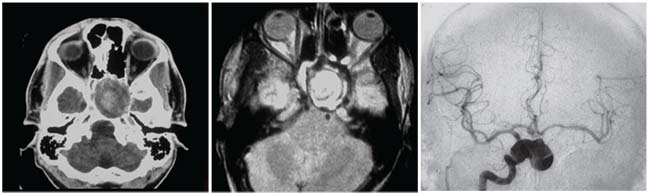 |
| This patient presented with slowly progressive visual loss in the left eye. Exam showed unilateral decreased visual acuity, relative APD, dyschromatopsia and a pale, cupped optic disc. CT scan (left) and MRI (middle) show a mass compressing the left optic nerve. A catheter angiogram (right) shows the lesion to be a large aneurysm coming off the right internal carotid artery. |
The reason for the discrepancy between these studies isn’t clear, but given the practical difficulties of neuroimaging so many individuals, the evidence probably doesn’t support neuroimaging all normal-tension glaucoma patients. On the other hand, when a patient presents with the kind of atypical findings we’ve been discussing in this article, neuroimaging that patient makes perfect sense.
Taking the Hint
Misdiagnosing neurologic disease can have profound consequences. However, sometimes it requires a leap of thinking to consider that unexplained vision loss may be the result of a neurologic problem. A glaucoma specialist I know had a patient who had been diagnosed with glaucoma 30 years earlier. About a year before I saw him he suffered dramatic vision loss, eventually reaching no light perception in one eye. He’d undergone an MRI 30 years earlier, and no problem had been detected; in addition, his recent visual field defects didn’t immediately suggest a neurological problem. For that reason, a retinal problem was the first possibility they considered.
As it turned out, retinal examination didn’t reveal any potential cause. The patient was also developing cataracts, so those were removed, but no improvement followed. The glaucoma specialist began to suspect that the patient was faking! Finally the patient was sent to our office, and we discovered a large pituitary adenoma. Once the adenoma was surgically removed, the patient’s vision returned to baseline.
The moral of the story is: When a glaucoma patient loses vision out of proportion to what you expect given the patient’s course or pressure, you need to consider other possibilities—including neurologic disease.
So, how do you decide when neuroimaging makes sense? The guiding principle should be taking note when the signs or symptoms are not what you’d expect. Is the patient getting worse for no apparent reason? If the pressure is 25 mmHg and it should be 10 and the patient is getting worse, you’re probably dealing with glaucoma. But if the pressure is 10 mmHg and it’s been 10 all along, and suddenly a new, different defect appears, you need to consider the possibility of a neurologic problem and consider neuroimaging the patient. REVIEW
Dr. Moster is a professor of neurology and ophthalmology at Thomas Jefferson University, and director of the neuro-ophthalmology fellowship at Wills Eye Hospital, in Philadelphia. He has no financial conflicts relating to anything discussed in this article.
1. Trobe JD, Glaser JS, Cassady JC. Optic atrophy. Differential diagnosis by fundus observation alone. Arch Ophthalmol 1980;98:6:1040-5.
2. Bianchi-Marzoli S, Rizzo JF 3rd, Brancato R, Lessell S. Quantitative analysis of optic disc cupping in compressive optic neuropathy. Ophthalmology 1995;102:3:436-40.
3. Tieger MG, Hedges III TR, Ho J, et al. Ganglion cell complex loss in chiasmal compression by brain tumors. Journal of Neuro-Ophthalmology 2017;37:1:7–12.
4. Ahmed II, Feldman F, Kucharczyk W, Trope GE. Neuroradiologic screening in normal-pressure glaucoma: study results and literature review. J Glaucoma 2002;11:4:279-86.
5. Greenfield DS, Siatkowski RM, et al. The cupped disc. Who needs neuroimaging? Ophthalmology 1998;105:10:1866-74.
6. Kesler A, Haber I, Kurtz S. Neurologic evaluations in normal-tension glaucoma workups: are they worth the effort? Isr Med Assoc J 2010;12:5:287-9.



