For example, a patient might present with advanced cupping and a pressure of 28 mmHg. My target pressure in this situation would be in the low teens with a fluctuation tolerance of less than 3 mmHg; but after using a prostaglandin the patient’s pressure might only go down to 21 mmHg, meaning that additional treatment is called for. (In this particular case adding a single agent probably wouldn’t suffice, so I would add a combination drug.)
Or, you might find that a patient has, in fact, reached your target intraocular pressure, but in follow-up visits the patient’s visual fields or optical coherence tomography scans—or both—are showing progression. In that case, you’ll need to lower your target pressure and add a second-line treatment. (It’s worth noting that simply switching the patient to a different prostaglandin seldom helps. If the patient is not responding adequately to one prostaglandin, there’s very little chance he’ll respond better to another.) Currently, about 30 percent of patients require adjunctive therapy within one year of starting prostaglandins.
| IOP Target Range* |
| Advanced glaucoma: Target range = low teens (least fluctuation tolerance, <3 mmHg) |
| Moderate glaucoma: Target range = mid teens (acceptable fluctuation <4 mmHg) |
| Mild glaucoma: Target range = high teens (acceptable fluctuation <5 mmHg) |
| *Canadian Society Guidelines and personal choice (after adjustment for pachymetry, if necessary) |
Today, when a first-line prostaglandin is not sufficient to stop progression, multiple alternatives are available to choose as an adjunctive treatment. However, different choices will produce better results with different patients. So when deciding which second-line treatment makes the most sense for a given patient, you need to consider a number of issues, including compliance concerns, possible side effects, allergic reactions, contraindications and the need for callbacks—as well as the possibility that a non-drug alternative like selective laser trabeculoplasty might be the most efficacious option.
Adding a Single-agent Drop
Let’s say you’re considering adding a second single-agent drop—usually a carbonic anhydrase inhibitor, beta-blocker or alpha agonist—to the patient’s regimen. Notably, any differences in the mean amount reduction in intraocular pressure you might achieve with the addition of a given agent should not be your main concern. More important considerations include the drug’s side-effect profile, dosing and nocturnal pressure-lowering efficacy.
One important note: Never add a second prostaglandin! This can sometimes result in an unexpected pressure rise so extreme as to send the patient to surgery. In fact, this so-called paradoxical pressure rise can even occur if someone simply uses the first prostaglandin more than once a day.2
Let’s take a closer look at the benefits and drawbacks of each of the other alternatives:
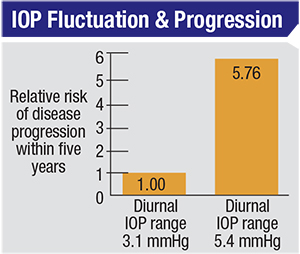 |
| Drug options that reduce IOP fluctuation may be advantageous to the patient because reducing fluctuation has been shown to reduce the risk of glaucoma progression. One study found that the hazard ratio between the higher and lower quartiles for “range in home IOP” was 5.76, even after adjusting for factors such as age, race, level of visual field damage at onset and IOP measured in the office.14 |
When combined with a prostaglandin, CAIs are generally better at lowering IOP than beta-blockers. And they are preferable as a second-line therapy for low-pressure glaucoma patients because they don’t cause systemic blood pressure to drop the way a beta blocker might. (If systemic blood pressure stays the same while IOP is lowered, one effect is an increase in the perfusion pressure of the eye, which is a good thing.) On the negative side, problems with CAIs include the risk of corneal endothelial toxicity. If a patient has Fuchs’ corneal dystrophy, for example, topical CAI drops could trigger corneal edema.
• Beta-blockers. The advantage of adding a beta-blocker such as timolol is that it can be used once daily in the morning. That means your patient only has to manage two drops: the beta-blocker in the morning and the prostaglandin at night. In contrast, if you prescribe a drop like dorzolamide or brimonidine, your patient will have to use it at least two or three times a day, in addition to the prostaglandin. Prescribing a less-frequently dosed beta-blocker increases the likelihood of compliance.
However, if you’re considering adding a beta-blocker to your prostaglandin, there are three things you should consider. First, does the patient have a history of emphysema or asthma? Beta-blockers are associated with a risk of bronchospasm, so this could be a contraindication.
Second, does the patient have a history of bradycardia or a bundle branch block (a defect of the bundle branches or fascicles in the electrical conduction system of the heart)? This can lead to trouble because beta-blockers can slow the patient’s heart rate. For example, if your patient is a runner, his resting heart rate could be in the low- to mid-50s. If you start the patient on a beta blocker, that rate could drop into the 40s, prompting the patient to have a fainting attack—and maybe causing another doctor to prescribe a pacemaker. Such a mistake is not unheard of, and often neither the other doctor nor the patient makes the connection. The patient will return to you and say,
“Oh, since I saw you I got a pacemaker.” If you ask why, the patients may say, “Well, my heart rate dropped from 55 to 42, and we don’t know why.” Then you realize that this was caused by the beta-blocker that you added; it pushed the patient into bradycardia. So, if you’re considering adding a beta-blocker to your patient’s prostaglandin, you have to get a good idea of the patient’s general systemic health, and in particular you have to investigate the patient’s resting pulse rate.
Other factors to consider include that adding a beta-blocker to a prostaglandin only causes a mild-to-moderate further decrease in pressure—less than you would likely achieve with either of the other two single-drug options. Finally, it’s worth remembering that tachyphylaxis may occur with beta-blockers, which means they may lose some of their effect after two to three years of use.
| Pros and Cons of Adjunctive Treatment Options | ||
| Pros | Cons | |
| Topical CAIs | • Significant lowering of IOP when combined with a prostaglandin. • Significant nocturnal pressure reduction. • Low allergy rate. • No effect on systemic blood pressure. With decreased IOP, this may increase ocular perfusion pressure, benefitting the optic nerve. | • Risk of corneal endothelial toxicity. |
| Beta-blockers | • Once-daily dosing | • Associated with risk of bronchospasm. • Can slow patient’s heart rate, potentially causing problems. • Less pressure reduction with a prostaglandin than other alternatives. • May lose some effect after two or three years of use. |
| Alpha agonists | • Possibly neuroprotective. | • High allergic response rate (about 28 percent). • Lowers systemic blood pressure, thus lowering ocular perfusion pressure, which may increase risk of progression. |
| Combination drops | • More aggressive therapy more quickly. • Fewer drops means better compliance. • The greater impact on IOP increases doctor confidence and patient adherence. • Fewer copays for patients. • Complementary mechanisms of action (in association with a prostaglandin). • Reduced exposure to preservatives. • Reduced washout effect. | • If result is not ideal, more work is required to determine the reason. • Multiple potential side effects. • Some insurance companies are withdrawing coverage. |
| Selective laser trabeculoplasty | • No issues with compliance. • No issues with side effects or allergy. • No drug costs for the patient. • Reduces the patient’s medication burden. • Reduces IOP fluctuation, associated with less risk of progression. | • Doesn’t work in every patient. • Can’t be done in some patients. • Some patients don’t like the idea of a laser treatment. • The effect eventually wears off (but the treatment can be repeated). |
It’s worth noting that a low perfusion pressure isn’t just an issue with low-pressure glaucoma patients. A few years ago Christina Leske, MD, published a review of the literature showing how low diastolic perfusion pressure impacts the prevalence and incidence of OAG. The data from multiple population studies showed that when diastolic perfusion pressure is less than 55, the prevalence and incidence of OAG increases two- to sixfold.7
Given this correlation, it’s reasonable to look for medications that might have the opposite effect—increasing perfusion pressure and hence improving the blood supply. A 2006 study by Luciano Quaranta, MD, showed the effects of various medications on ocular perfusion pressure.8 He demonstrated that only prostaglandins and CAIs increase perfusion pressure and improved the blood supply because they lower eye pressure without decreasing blood pressure. In contrast, alpha agonists like brimonidine were associated with a significant decrease in perfusion pressure. (Perfusion pressure was unchanged with beta-blockers because the impact of the beta-blockers was negligible at night.)
Adding a Combination Drop
Recently, it’s become more common for glaucoma patients to receive a fixed-combination product as the initial adjunctive therapy.9 Instead of the doctor adding a CAI to the prostaglandin, and later adding timolol and eventually brimonidine, people are just going straight to the combination therapy once a prostaglandin has been deemed insufficient.
There are a number of reasons that this may be advantageous:
• Combination drops provide more aggressive therapy more quickly. As long as the combination agent has no negative side effects, this may reduce the pressure sooner and to a greater degree.
• Combination drops may improve treatment adherence. Instead of dealing with three or four bottles, the patient only has to manage two bottles. Improved adherence means more stable IOP control over time.
• The more rapid and dramatic impact of combination drops makes the physician more confident and the patient more adherent to the protocol. The dramatic IOP reduction that’s usually achieved with a combination drug instills confidence in the treating physician, and that, in turn, has a positive effect on patient compliance. I’ve seen this many times. After adding the combination drug, the patient comes in with a significant reduction in IOP, boosting his confidence in the doctor and his motivation to continue taking the drops on a regular basis. The patient can see that the treatment is really working. In contrast, if the effect of the adjunct is not great, patients often lose enthusiasm for following the treatment regimen.
• Patients have fewer copays. Each time the doctor adds a drug the patient has to come in for an additional visit and pay another copay. Adding multiple drugs in a single drop means fewer copays for visits and drugs, and fewer pressure-check visits (unless the patient has an allergic reaction).
• Combination drugs may provide better pressure control because of complementary modes of action. The prostaglandins are great outflow-enhancing drugs. All of the combination drugs available in the United States are inflow-inhibitors, so they complement the prostaglandins. This means great diurnal control and more consistent lowering of pressure during the day.
• Reduced exposure to preservatives. If you put two drops in instead of four, you’re going to reduce the amount of preservative placed on the eye.
• Reduced washout effect. The reality is that when taking multiple drops at the same time of day, very few patients wait five minutes between the drops. Thus, there is invariably a washout effect. If the multiple drugs are all in one drop, that’s no longer an issue.
• Fewer doses for the patient to manage. Many of these combination drugs are still twice-a-day dosing, so the patient doesn’t have to manage drops at three different times during the day.
Of course, there are a few potential downsides to adding a combination drug. One issue is that if the added drop fails to produce the pressure reduction you expected—or if the combination drop triggers an allergic reaction—you won’t know which drug was responsible. That means eliminating one of the components of the combination drug to determine which is triggering the allergic reaction, and/or which drug is not working for this patient. Having to do a process of elimination removes all the benefits that come with using a combination drug. (Luckily, this doesn’t happen too often. Allergic reactions will probably only occur in 5 to 10 percent of patients receiving a combination drug.)
Another potential concern is that you’re exposing the patient to the side effects of two types of drugs. However, you know the side effect profiles of each component of a combination drop, so if a problem occurs you should be able to identify the culprit immediately. (Also, less-problematic side effects would still have to be tolerated if you gave the patient the drops separately.)
One final issue has just become a concern in the past few months. Some insurance companies have begun refusing to pay for combination glaucoma drops such as generic Cosopt or Combigan—drops they covered in 2015. This is forcing us to shift patients back to multiple single-medication drops, simply because of the insurance companies’ change in policy. The reason for this abrupt change in policy isn’t clear, but we’re getting a lot of phone calls about it. Now, patients who are stable on a combination drug have to change their routine and manage more bottles.
What About SLT?
Another option, if a first-line prostaglandin is not sufficient, is selective laser trabeculoplasty. SLT uses laser energy to cause alterations in the trabecular meshwork that result in a fairly long-lasting increase in outflow. Not surprisingly, improving outflow with a laser instead of additional drugs reduces issues with compliance, allergies, side effects and cost. When SLT is combined with a prostaglandin, the patient remains
on monotherapy.
Typically, patients who benefit the most from SLT are the young and the elderly. Younger patients (in their 40s and 50s) usually have a busy life and don’t understand the full import of glaucoma, and therefore don’t appreciate the need for compliance. At the other end of the spectrum, patients in their 80s and 90s may have memory issues and arthritis, and their medication burden may be considerable. They’re often very grateful to have the number of medications they have to use reduced.
| Given its many advantages, I generally offer SLT as a second-line option to most patients who need an adjunctive therapy. |
Reducing the amount of fluctuation is important because pressure fluctuation has turned out to be a significant factor in glaucoma progression. For example, a glaucomatous eye with a pressure fluctuation of about 5.5 mmHg has a six-times greater risk of progression than an eye that has a fluctuation of 3 mmHg.14 And while this factor is important in any type of glaucoma, controlling fluctuation is absolutely vital in low-pressure glaucoma. I have found that controlling fluctuation in low-pressure glaucoma patients stabilizes their disease.11 In such patients, I typically recommend considering SLT as a second-line treatment rather than another drug, because my goal in that situation is not so much to lower the mean pressure as to control pressure fluctuation.
Given its advantages, SLT is potentially a good choice for many patients. Nevertheless, it does have several limitations. First, it doesn’t work in 15 or 20 percent of the patients we try it on, for reasons we don’t yet understand.
Second, there are patients in whom you cannot do SLT, such as patients with uveitic glaucoma and some patients with trauma-associated glaucoma (there’s a good chance it won’t work if the trabecular meshwork has been injured in the past). Third, some patients simply don’t like the idea of a laser being pointed into their eye. Fourth, the effect doesn’t last more than two or three years. (Luckily, it can be repeated.)
Despite these limitations, given its many advantages, I generally offer it as a second-line option to most patients who need an adjunctive therapy. It avoids the downsides of adding drugs and is better in terms of its impact on the patient’s quality of life.
Choosing the Best Option
When choosing a second-line treatment to add to a prostaglandin, you should consider its potential side effects; the general systemic health of the patient, especially in terms of bronchial and heart issues; the patient’s ability and willingness to adhere to your protocol; the cost to the patient; how the adjunct will affect the patient’s IOP fluctuation; how it will affect the patient’s diastolic ocular perfusion pressure; and how managing multiple medications (and possibly having to return to your office) will impact the patient’s quality of life. If you choose to prescribe a second drug, you also need to be on the lookout for any allergic or systemic-health reaction.
Today, we are fortunate to have multiple second-line alternatives to choose from (with more potential options such as rho-kinase inhibitors in the pipeline). That gives us the opportunity to help our patients with the fewest possible downsides. We just have to choose wisely.
Dr. Asrani is a professor of oph-thalmology at Duke University School of Medicine and a specialist in the Glaucoma Service at Duke Eye Center in Durham, N.C.
1. Damji K, Behki R, Wang L. Canadian perspectives in glaucoma management: Setting target intraocular pressure range. Can J Ophthalmol 2003;38:3:189-97.
2. Herndon LW, Asrani SG, Williams GH, Challa P, Lee PP. Paradoxical intraocular pressure elevation after combined therapy with latanoprost and bimatoprost. Arch Ophthalmol 2002;120:6:847-9.
3. Nakamoto K, Yasuda N. Effect of concomitant use of latanoprost and brinzolamide on 24-hour variation of IOP in normal-tension glaucoma. J Glaucoma 2007;16:4:352-72.
4. Guedes G, Karan A, Mayer H, Shields M. Evaluation of adverse events in self-reported sulfa-allergic patients using topical carbonic anhydrase inhibitors. J Ocular Pharc and Therap 2013;2:5:456-61.
5. Liu C, Cheng C, Ko Y, Hsu W. Diurnal intraocular pressure and blood pressure with two dosing regimens of brimonidine in normal tension glaucoma. J Chin Med Assoc 2004;67:9:465-71.
6. Krupin T, Liebmann JM, Greenfield DS, Rosenberg LF, Ritch R, Yang JW; Low-Pressure Glaucoma Study Group. The Low-pressure Glaucoma Treatment Study (LoGTS) study design and baseline characteristics of enrolled patients. Ophthalmology 2005;112:3:376-85.
7. Leske, MC. Ocular perfusion pressure and glaucoma: Clinical trial and epidemiologic findings. Curr Opin Ophthalmol 2009;20:2: 73-78.
8. Quaranta L, Gandolfo F, Turano R, Rovida F, Pizzolante T, Musig A, Gandolfo E. Effects of topical hypotensive drugs on circadian IOP, blood pressure, and calculated diastolic ocular perfusion pressure in patients with glaucoma. Invest Ophthalmol Vis Sci 2006;47:7:2917-23.
9. Schmier JK, Hulme-Lowe CK, Covert DW. Adjunctive therapy patterns in glaucoma patients using prostaglandin analogs. Clinical Ophthalmology 2014:8:1097-1104.
10. Prasad N, Murthy S, Dagianis J, Latina M. A comparison of the intervisit intraocular pressure fluctuation after 180 and 360 degrees of selective laser trabeculoplasty (SLT) as a primary therapy in primary open angle glaucoma and ocular hypertension. J Glaucoma. 2009;18:2:157-60.
11. El Mallah M, Walsh M, Stinnett S, Asrani S. Selective laser trabeculoplasty reduces mean IOP and IOP variation in normal tension glaucoma patients. Clinical Ophthalmology 2010:4;889-893
12. Lee J, Gangwani R, Chan J et al. Prospective Study on the Efficacy of Treating Normal Tension Glaucoma With a Single Session of SLT. J Glaucoma. In Press.
13. Tojo N, Oka M, Miyakoshi A, Ozaki H, Hayashi A. Comparison of fluctuations of intraocular pressure before and after selective laser trabeculoplasty in normal-tension glaucoma patients. J Glaucoma 2014;23:8:e138-43.
14. Asrani S, Zeimer, R, Wilensky J, Geiser D, Lindenmuth K, Vitale S. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. Journal of Glaucoma 2000;9:2:134-142.



