Just when the clinician thinks glaucoma can't get any more challenging to manage, a patient presents with glaucoma and a co-existing condition that makes things even more difficult. For example, a patient may have uveitis that needs to be diagnosed and controlled before the pressure can be definitively addressed, or a central retinal vein occlusion may have induced neovascular glaucoma. In this article, glaucoma specialists share advice on several of the conditions the comprehensive ophthalmologist is most likely to encounter alongside, or even as a cause of, glaucoma.
Uveitic Glaucoma
Though not common, uveitic glaucoma can be very challenging to treat, since the mainstay of anti-inflammatory treatment, steroids, are also well-known for increasing intraocular pressure. In addition, properly diagnosing the cause of the inflammation can be an involved process, as well.
"Not infrequently, the patient will have a lower IOP than you'd expect initially," says Jeffrey Henderer, MD, chair of ophthalmology at
Clinicians first try to calm down the inflammation in the hope that it will cause the IOP to decrease. Doing this, however, involves accurately diagnosing the cause of the inflammation.
Glaucoma specialist Steven Simmons, MD, of
"The most common cause in the patient who presents with uveitis and high pressures is herpes simplex and herpes zoster," he continues. "If the uveitis is unilateral, also think about Fuchs' heterochromic iridocyclitis. The other presentation that's relatively common is the patient who's HLAB27-positive with anterior uveitis, which tends to appear as a very fibrinous reaction and can also occasionally present with pupillary block. Such patients can have dramatic elevations in their pressure."
Based on the patient's history and clinical presentation, possible causes of the uveitis include:
• adult or juvenile rheumatoid arthritis;
• ankylosing spondylitis;
• herpes;
• Behçet's disease;
• Fuchs' heterochromic iridocyclitis;
• reactive arthritis;
• sarcoidosis;
• tuberculosis; and
• Lyme disease.
Depending on what the clinician suspects as the etiology, physicians say helpful lab tests include:
• herpes zoster or herpes simplex titers (if it's not the classic zoster in which there is nerve involvement or skin lesions);
• rheumatoid factor blood test and antinuclear antibody test for rheumatoid arthritis;
• erythrocyte sedimentation rate;
• tuberculosis skin test;
• angiotensin converting enzyme blood test and chest X-ray for suspected sarcoidosis;
• HLAB27 screening; and
• Lyme titers.
James Tsai, MD, Robert R. Young Professor and chair of ophthalmology at
After he's got a handle on the cause of the inflammation, Dr. Simmons then tries to determine what might be behind the pressure rise. "You may be dealing with an open-angle process or an angle-closure process related to the uveitis," he says. "Angle closure can be caused by a pupillary block mechanism, bridging of keratotic precipitates causing anterior synechiae in sarcoidosis, or from fibrin collecting in the inferior angle, causing sludging."
Dr. Tsai's typical approach to quieting the inflammation in non-infectious cases is topical prednisolone acetate q1h or q2h while awake for one to four days, at which point the patient returns for an exam. He then gradually tapers the steroid as the inflammation subsides. He says he'll increase the steroid to pre-taper levels if the inflammation returns. If he determines the cause is infectious, Dr. Tsai will treat the patient aggressively with antibiotics first. Dr. Tsai says it's a good rule of thumb to quiet the inflammation before attacking the glaucoma. However, if the uveitis patient shows damage to the optic nerve or the eye pressure is very high, he'll initiate glaucoma therapy, typically with a topical aqueous suppressant.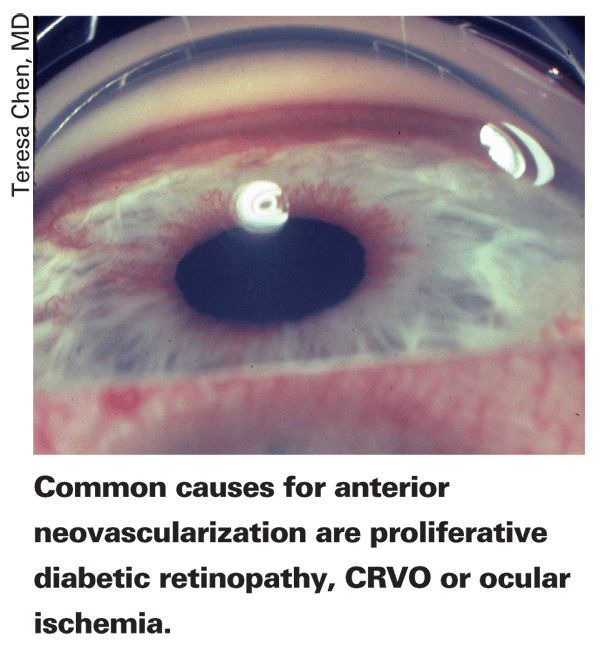
In certain cases, such as some patients with herpes and patients who still retain some angle function, doctors say the IOP will decrease when the inflammation is treated. In others, however, the treatment becomes more challenging, since their pressure stays elevated after the inflammation is resolved. "The question then is, are the steroids causing the pressure rise or has there been some trabecular meshwork dysfunction from the inflammation that's causing the rise?" says Dr. Henderer. This is where the steroid tapering can help. "If the pressure stays elevated after the taper, you're left with using glaucoma medications, namely topical, or even oral, aqueous suppressants," he says. "The prostaglandin analogs are thought to be pro-inflammatory, and, as such, are probably not your best choice."
In weeding out the steroid responders, Dr. Simmons says that most of them will have a family history of glaucoma. "If someone is having a steroid response, turn to the rheumatologist for help in suppressing the underlying systemic condition. He may be able to use other immunosuppressives without having to rely solely on topical or systemic steroids."
Dr. Tsai says that, in patients with secondary angle-closure glaucoma due to their uveitis, posterior synechiae can develop and obstruct the flow of aqueous from the posterior chamber to the anterior chamber, thereby causing pupillary block. Peripheral anterior synechiae may also develop and lead to increased IOP. When a patient has 360-degree posterior synechiae and secluded pupil, Dr. Tsai advises that laser iridotomy will often relieve the pupillary block and control the IOP.
For patients whose pressure is uncontrollable by first-line responses, Dr. Henderer turns to surgery. "The decision between trabeculectomy and a tube shunt is driven mainly by the inflammatory status of the eye," he says. "If the eye is quiet and the pressure is chronically elevated, I tend to go with a trabeculectomy with mitomycin-C. If the eye's not particularly quiet, or I can't get it as quiet as I'd like, I typically go with a shunt. Also, if the inflammation will likely flare up again, I'd probably opt for the shunt."
Dr. Henderer recommends performing an iridectomy during surgery on the uveitic glaucoma patient. "It can prevent acute angle closure if the eye develops posterior synechiae, which often form," he says. "And, if you're implanting a shunt, you can also put the tube tip in the iridectomy so the iris doesn't obstruct the tube."
Dr. Simmons will also implant a seton in a pseudophakic patient in whom he expects a fair amount of inflammation. However, for a younger patient with good vision in both eyes, he'd opt for a trabeculectomy with antimetabolites. "I'm sure there's some controversy here, but I'm not a big fan of putting setons in a phakic patient with no history of previous surgery," he says. "This is especially true if the patient has good vision in both eyes, because you can get into motility problems from a seton. Taking someone with ocular inflammation and giving him or her double vision will not make a happy person.
"In the case of the inflammatory glaucoma patient in whom I'm performing a trabeculectomy, I use the antimetabolite for a longer duration than my usual two and a half minutes," Dr. Simmons continues. "I tend to use a concentration of 0.2 mg/cc."
Pseudoexfoliative Glaucoma
Pseudoexfoliation often occurs alongside glaucoma, accounting for up to 20 percent of open-angle glaucoma cases.1 Of the patients with pseudoexfoliation, a fifth will present with glaucoma and elevated pressure, but 15 percent more will develop glaucoma within 10 years.1 Dr. Henderer notes that the pseudoexfoliation patient tends to be older, and the condition often presents asymmetrically, though, in general, it's ultimately a bilateral disease.
Though pseudoexfoliation is a relatively common cause of glaucoma, Dr. Henderer says it's still possible to miss it. "It can be missed unless you look closely at the edge of the pupil for the hallmark white, almost dandruff-like, material," he says. "Also, the pupil should be dilated to look for material on the surface of the lens, typically in a bull's-eye configuration. It may also be hard to detect elevated pressure because IOP can fluctuate in these patients. Wide swings in pressure are possible."
Atlanta
Dr. Simmons also advises physicians to "perform gonioscopy to make sure the angle isn't narrow and the patient doesn't need a peripheral iridectomy, as these eyes tend to have their lens-iris diaphragm shifted forward a little."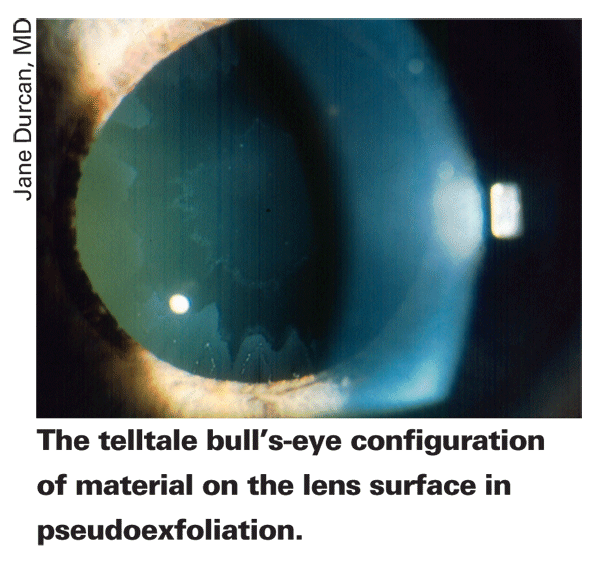
If you diagnose a patient with pseudoexfoliation but not glaucoma, it's important to perform baseline tests, namely visual fields and optic nerve photos, and then simply see the patient once a year. "We always have to balance safety with what's reasonable," says Dr. Brown. "We don't want to harass the patient just because he's unfortunate enough to have pseudoexfoliation. Visits are once per year, but if the patient has a relatively high pressure, such as 20 or 21 mmHg, and his optic nerve looks suspicious, I'll see him in six months."
The physicians to whom we spoke say they treat pseudoexfoliative glaucoma in a manner similar to chronic open-angle glaucoma, usually starting with a prostaglandin analog and increasing therapy if the glaucoma remains uncontrolled.
"My maximal medical therapy, for patients who need it, would probably be four drops: a prostaglandin; a beta-blocker; an alpha-agonist and a carbonic anhydrase inhibitor," says Dr. Henderer. "I'll use oral CAIs as needed to help with acute pressure control issues, but I tend to shy away from them for maintenance of pressure control if I can, mainly because of side effects."
Pigmentary Glaucoma
In a case of pigment dispersion, pigment is freed from the back of the iris as it rubs on the zonules with dilation and constriction of the pupil. About a quarter to half of patients with the condition go on to develop glaucoma.2
"The liberated pigment deposits on the angle and creates a heavily pigmented trabecular meshwork and slit-like transillumination defects in the mid-periphery of the iris," describes Dr. Henderer. "The third part of the diagnostic triad is Krukenberg's spindle, which is a pigment deposition on the corneal endothelium; it appears as a vertical stripe of pigment in the lower portion of the central cornea.
"These findings can be easy to miss if you don't look for them," he continues. "Sometimes, the Krukenberg's spindle can be subtle. Pigmentation in the meshwork can be hard to appreciate unless you look at the fellow eye on gonioscopy to assess the difference between the two eyes. And the slit-like defects are tough to see unless you transilluminate the iris. The transillumination defects, if present, can be really difficult to see in eyes with dark irides." He says the eyes are typically myopic, have a deep anterior chamber with a concave iris configuration and that the disease is usually symmetrical on presentation. Physicians say the condition tends to occur in younger people, in contrast to pseudoexfoliation, which is more prevalent in older patients.
The mechanism by which pigment dispersion causes glaucoma appears to be a reverse pupillary block. "The peripheral iris appears to be bowing back and rubbing on the zonules, releasing iris pigment into the anterior chamber where it accumulates in the angle and is phagocytized there," says Dr. Simmons. "Though this material can drive up the pressure like coffee grounds clogging a sink, there's clearly more to it than that, as there are many patients with pigment dispersion who never develop glaucoma."
For the pigment-dispersion patient without glaucoma, follow-up is similar to that used for pseudoexfoliation patients. "The person needs baseline fields, optic nerve photos and annual visits after that," says Dr. Simmons. "If he has elevated IOP, then he's seen more frequently. In the initial baseline studies, you may pick up some subtle asymmetry. You may see some asymmetry in the neural rim area when you examine it with the Heidelberg Retinal Tomograph, some early nerve fiber layer loss in one eye over another with optical coherence tomography or the Zeiss GDx, or a little decreased neural retinal sensitivity with matrix fields, SITA/SWAP or white-on-white perimetry."
Another possible risk factor for the development of glaucoma in the pigment-dispersion patient is physical exertion. "There's some evidence that when people with pigment dispersion exercise, they can get a pressure spike," says Dr. Brown. Dr. Simmons agrees, and will make it a point to discuss this risk with his patients. "I had one patient with pigmentary glaucoma who presented in the emergency room like an acute angle-closure patient with pressure in the 60s," Dr. Simmons recalls. "He had just finished exercising and had this florid pigment release that drove up his pressure. Some individuals with pigmentary disease will see halos around lights after exercise. So, you need to discuss this aspect of the disease with your patients and determine if they notice any blurred vision after exercise. The one instance where I'd do an iridectomy in pigmentary glaucoma is on the patient who exercises and notices that his pressure is driven up afterward as a result of a lot of pigment being released."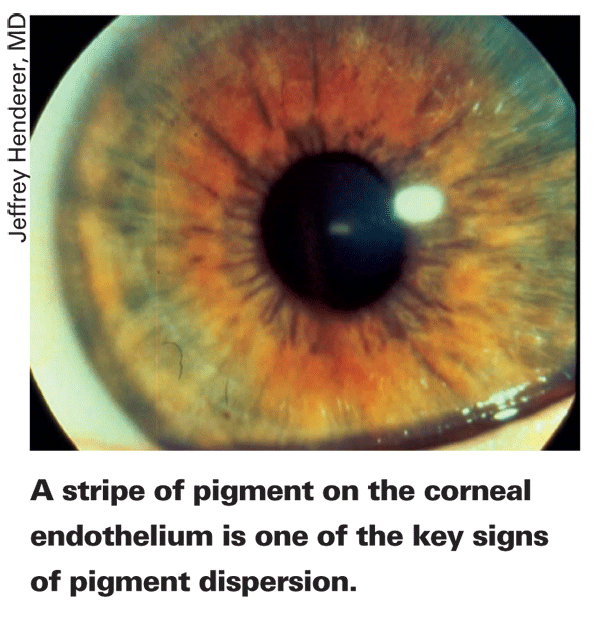
The surgeons to whom we spoke initiate medical therapy for the pigmentary glaucoma patient, and will then increase therapy accordingly to control the disease. Dr. Simmons says he doesn't think laser iridectomy is effective in these patients, though filtration surgery is an option for those with aggressive disease that doesn't respond to medication.
Neovascular Glaucoma
This secondary glaucoma can spring from a host of conditions. "It can be very challenging because patients often present with markedly elevated pressure," says Dr. Tsai. "And the physiologic mechanisms that cause the angiogenesis are complex."
Though it can arise from a number of disease states, physicians report that the conditions most often behind the anterior neovascularization are central retinal vein occlusion, proliferative diabetic retinopathy, ocular ischemic syndrome and central retinal artery occlusion. If the patient isn't diabetic or has no history of CRVO, surgeons say it's a good idea to order a carotid ultrasound, a carotid flow study, a computed tomography angiogram or magnetic resonance angiogram and possibly a carotid endarterectomy to rule out ocular ischemic syndrome.
"In most cases, you're faced with a patient with a pressure in the 60s and a steamy, swollen cornea and pain due to the elevated pressure," says Dr. Henderer. "At that point, you have to try to control the pain and reduce the pressure. A treatment with Avastin or Lucentis and panretinal photocoagulation can dry up the vessels, which is helpful, but won't necessarily fix the pressure problem if the angle is already closed. Most clinicians would use topical glaucoma drops and possibly even oral medications like Diamox, as well as Pred Forte and atropine, which can be very useful for pain control in these eyes. You can then decide if surgery would be helpful. For me, if the eye has reasonable vision potential and lowering the pressure will therefore preserve the vision, a tube shunt is a great choice. If it's an eye with very poor vision potential, then your goal is really pain control."
Managing the patient is a two-person job, surgeons say, with you collaborating with a retina colleague, who will usually be the one administering the anti-VEGF treatment, performing the PRP and otherwise managing the retinal condition, while you handle the glaucoma.
When considering surgery, Dr. Simmons feels that any neovascular glaucoma patient with 5/200 vision or better deserves a seton. "I've had patients who had a vein occlusion, were treated and then had that eye become their 'bad' eye," he recalls. "But then they developed a vein occlusion in the fellow eye, and it had a worse outcome—their previous 'bad' eye had become their good eye. So, I wouldn't be too quick to write off a 20/400 eye due to a vein occlusion, because the patient may have underlying vascular disease that will make the other eye the worse eye in the end." He recommends avoiding non-steroidal anti-inflammatories for pain control, to avoid affecting platelet aggregation should the patient need surgery.
The ideal situation would be to catch the patient before the angle closes. "If you have the opportunity, and it doesn't present itself as often as you'd like, it would be best to try to prevent the patient from developing angle closure," says Dr. Henderer. "If you see the patient and diagnose the neovascularization of the iris or angle prior to the angle becoming closed, then it's possible, with treatments such as Avastin and PRP, to prevent the angle from closing. Then, you've got a fighting chance to keep the pressure from becoming elevated."
Surgical Considerations
There are a couple of caveats to keep in mind if you have to perform glaucoma surgery on patients with these secondary glaucomas. "Both neovascular and uveitic glaucoma aren't considered to be ideal for long-term trabeculectomy survival," says Dr. Henderer. "This is because of the healing that can occur due to the pro-inflammatory state of the eye. Neovascular glaucoma can be challenging because when you actually enter the eye you can cause some hemorrhage by cutting the vessels in the angle and causing a hyphema.
"Also, both uveitic and neovascular glaucoma can occasionally have a problem with the iris wrapping around the tube of an implant and blocking it," Dr. Henderer says. "Fortunately, in my experience, that's pretty rare. Also, if you perform surgery in the setting of very high pressure, too rapid of a postop pressure drop runs the risk of choroidal detachment or suprachoroidal hemorrhage.
"For neovascular glaucoma," Dr. Henderer continues, "I often can't do an iridectomy because of the risk of causing a hemorrhage when I cut the iris. So, in that case, if it's a pseudophakic eye with poor vision and a closed angle, often there is a very deep posterior chamber, and it's not unreasonable to put the tube in the posterior chamber in front of the intraocular lens with the tube sticking out into the pupil. This helps minimize the risk of corneal decompensation and also helps prevent the tube from being incarcerated by the iris."
Dr. Simmons also makes tube placement a priority when implanting a seton in the neovascular glaucoma patient. "I try to place the tube in a position where I'm not going to get bleeding," he says. "It's important to do preop gonioscopy to examine the area in which you plan to put the seton and see whether the clock hours have a lot of peripheral anterior synechiae and whether there's still active neovascularization there. You want to avoid those areas. Also, you'd really like it if the iris neovascularization is controlled or gone when you put the tube in; otherwise there's a higher incidence of postop hyphema."
Dr. Henderer says a particular suture technique for trabeculectomy can help avoid a too-rapid pressure drop postop. "I use a combination of permanent and releasable sutures to lower the pressure in a stair-step fashion," he explains. "I put in permanent sutures that adjust the pressure for what I'd eventually like the long-term pressure to be, but I temporarily tighten the trabeculectomy flap with releasable sutures so the pressure is at an intermediate point between the preop pressure and the long-term postop goal pressure. So, if the IOP was 45 mmHg preop and I'd like the eventual pressure to be 15, I'd set the permanent sutures for 15 and the releasable suture for an intermediate pressure of 25 to 30. That way, after a week, I can pull the releasable sutures and minimize the trauma to the eye as the pressure goes down, stepping it down in a way that I hope will be safer.
"In a similar vein," Dr. Henderer continues, "I'm reluctant to use Ahmed valves for very high pressure eyes, because when you put in an Ahmed valve, the pressure is immediately 5 to 8 mmHg the next day. If the pressure goes from 55 down to
5 mmHg, that's a high-risk scenario for choroidal detachment or suprachoroidal hemorrhage. So, I generally favor a Baerveldt shunt, because even though the shunt isn't particularly titratable, at least when you tie off the tube you can put a slit or two in it to allow some aqueous to flow up the tube. This doesn't open the tube completely, so it yields a bit more of a stair-step pressure control over the first few weeks postop until the tube opens. It doesn't solve all of your problems, unfortunately, and, occasionally, the slits don't work and the pressure does drop from 55 to 5 when you open the tube or it opens by itself in a month. Generally speaking though, in my experience it's safer than having an immediate pressure drop the day after the surgery.
"If I'm faced with an eye that needs immediate pressure control and I'm using a Baerveldt shunt, I'll often perform a simultaneous non-antimetabolite trabeculectomy," says Dr. Henderer. "This provides some pressure control immediately postop and when the trabeculectomy scars closed, the tube is about ready to open."
Though these secondary glaucomas can be challenging, experts say that vigilance and prompt treatment can help fight these double-threats.
1. Samuelson T, Shah G. Pseudoexfoliative glaucoma. In: Yanoff M, Duker J. Ophthalmology.
2. Ritch R, Steinberger D, Liebmann JM. Prevalence of pigment dispersion syndrome in a population undergoing glaucoma screening. Am J Ophthalmol 1993;115:707-10.



