Visiometrics says that the HD Analyzer’s visual
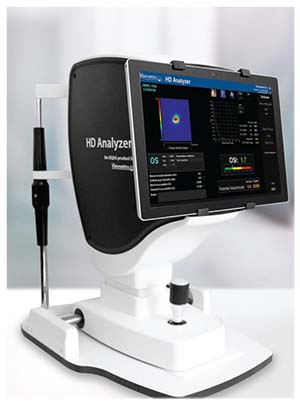 |
The Touch Screen Package includes a Surface Pro tablet that comes loaded with Visiometrics HD Analyzer software, screen holders and an installation kit. It can be purchased with a new HD Analyzer system or as an upgrade to an existing system. It is currently only available to users in the United States.
For more information on Visiometrics’ Touch Screen update, visit visiometrics.com/shop.
ThromboGenics Introduces ‘Already-Diluted’ JETREA
In late September, ThromboGenics released a new formulation of JETREA in the United States designed to eliminate the preparatory dilution steps before injection. At the point of administration into the eye, the strength, potency, composition and pharmaceutical form of the already-diluted formulation remain identical to the currently available formulation after dilution, the company says.
JETREA is the first and only pharmacological treatment in the United States for symptomatic vitreomacular adhesion. The product was introduced in 2013 and has been used to treat close to 30,000 patients globally.
ThromboGenics also announced that it regained the full global rights to JETREA from Alcon. In an announcement of the transaction, the company says that it was based on a mutual agreement that the unique characteristics of JETREA make ThromboGenics a “better fit for building a sustainable long-term business.”
Following the agreement with Alcon/Novartis, ThromboGenics aquired cash that it will use to support its pipeline of novel and unique disease-modifying medicines for the treatment of diabetic eye disease.
For more information on this new formulation of JETREA, visit jetrea.com.
Zeiss Launches CLARUS 500
In late September, Zeiss announced the launch of its CLARUS 500 ultra-widefield fundus imaging system. Zeiss says that the new color system can aid in the diagnosis and documentation of ocular disease, ensuring confidence when evaluating the optic disc, nevi and lesions, when the color is important.
Zeiss says that the CLARUS 500 can capture a high-resolution image down to 7 µm with the ultra-widefield retinal camera. It also captures high-resolution fundus autofluorescence images in blue and green and infrared, as well as external eye images. The CLARUS 500 produces a 133-degree HD widefield image. These images are automatically merged to achieve a 200-degree ultra-wide field of view, allowing clinicians to easily review and compare high-quality images captured during a single exam.
Zeiss claims that the CLARUS 500 produces images in true color that closely resemble the coloration of the retina as seen through direct observation during clinical examination. This accurate coloration and resolution is important for evaluating focal change in rim tissue, nerve pallor, dry age-related macular degeneration RPE pigment changes and drusen.
For more information on Zeiss’ Clarus 500, visit zeiss.com/meditec/us/products/ophthalmology-optometry/retina/diagnostics/fundus-imaging/clarus-500.html.
The FaceDownWalker
In hopes of alleviating his wife’s post-vitrectomy discomfort, Wayde Faust, an inventor based in California, invented the FaceDownWalker, a mobility device that he says allows patients recovering from vitrectomy to stand, walk around and live a more active lifestyle, without risk of retinal detachment.
The FaceDownWalker transfers the weight of the
 |
Mr. Faust’s wife began using the walker after her own surgery. “When my wife was first told she would have to keep her head parallel to the floor for up to 10 days, depression and claustrophobia set in,” he says. “I hated seeing her like that. So I came up with the FaceDownWalker. Once we got her in it, and I held her hand to lead her around the house, her attitude completely changed. It really did make a big difference during the recovery process.”
For more information and testimonials on the FaceDownWalker, visit facedownwalker.com.
NIDEK Launches the TS-310 Tabletop Refraction System
NIDEK recently announced the U.S. launch of its new tabletop refraction system that integrates chart and refractor into a single unit. Nidek says the unit’s compact, minimalist design allows easy installation and assimilation in the office.
The refraction system has a triple-performance combination of control box, refractor and chart unit. The control box has a 5.7-inch color LCD touch screen for ease of use. NIDEK says the product’s compact design—it measures from 40 to 70 cm wide and 50 cm deep—allows for its integration into many offices.
The TS-310 uses the same high-resolution charts for both far and near testing. The clarity of the LCD allows measurement of visual acuity at 5 m and near visual acuity at 40 cm with the same accuracy as at the actual distances.
NIDEK also claims that because of the unit’s compact design, it allows for either standing or sitting examinations, as well as examinations from either the right or left side of patients. The system doesn’t require complex angle and distance adjustments, allowing any staff member to use it without expert knowledge.
For more information on NIDEK’s TS-310, visit nidek-intl.com/product/ophthaloptom/refraction/ref_optometry/ts-310.html.
Bausch + Lomb’s EnVista MX60E Intraocular Lens
Bausch + Lomb recently introduced the next-generation hydrophobic acrylic lens, providing improved material properties to enhance optic recovery following delivery.
The MX60E is a hydrophobic, acrylic, advanced-optic IOL with StableFlex technology designed to provide controlled unfolding, excellent stability and improved optical recovery. It also features AccuSet haptics, which provide extensive interaction with the capsular bag to aid in securing the lens position through its offset design and broad contact angle, the company says.
With this new addition, the enVista remains the first and only single-piece, hydrophobic acrylic IOL domestically available. Bausch + Lomb also claims that the lens has the potential for increased resistance to scratches and abrasions that may occur during loading and surgical manipulation. The company says its aberration-free design provides for excellent visual outcomes in a wide range of patients.
For more information on the MX60E IOL, visit bausch.com/our-company/recent-news/id/2383/10162017-monday.



