While many have adopted the phrase “bench-to-bedside research” as a pseudonym for translational science, few projects ever begin de novo with the aim of spanning that entire distance. And while recruiting basic scientists to pursue more clinically relevant projects is key, attracting clinicians to projects that integrate basic science with therapeutic development and application is just as important.3 It strikes us that we’ve been doing just this for some time, and it’s this intersection of science and medicine that has formed the basis for 250 installments of “Therapeutic Topics” over the past 20 years (see “The Roots of Translation,” on the opposite page).
This month, we consider one example of applying a translational approach, and describe how we have followed a continuous thread of experimental questions and answers focused on blinking and its relationship to ocular surface disease. We discuss how this knowledge has advanced clinical thinking and refine endpoints in clinical trials. This retrospective describes a 40-year mission based on simply following the physiology with a dogged determination that reminds us of a line from Tennyson’s Ulysses: “To follow knowledge like a sinking star, beyond the utmost bound of human thought.”
Blink Biology in the Clinic
The process of reflex and conscious blinking has always been understood to be an essential element of visual function, but most research prior to the past few decades was focused on psychological and behavioral aspects of blinking.
One of our first observations on blink made in the clinic was noting that superficial punctate keratitis was often located in the inferior crescent of the palpebral fissure.4 Following up on this, we videotaped 10 patients and 10 controls, and found in subjects with keratopathy a consistent pattern of incomplete blinking that left this exposed region of the cornea scattered with puncta. While both groups showed a similar blink rate (16 to 17 blinks/min.), blinks were complete (where the upper lid makes contact with the lower lid) in 80 percent of the normal group, and in only 7.5 percent in patients with SPK. This suggested that the ocular surface pathology, SPK in this instance, was secondary to a primary blink abnormality rather than the keratitis modifying blink secondarily. This modest clinical finding of 1976 became the starting point for a thread that has woven through our research as it made its way into the lab, out to the clinic and back again several times over.5-13
|
Recognition of the intricate nature of these two metrics led to the development of the ocular protection index.8 The ratio of tear-film breakup time to another metric, inter-blink interval, provides an instantaneous snapshot of ocular surface health. When patients have OPI scores less than 1, i.e., TFBUT is less than IBI, the ocular surface is exposed. When patients have OPI scores greater than 1, i.e., TFBUT is greater than IBI, the tear film remains stable between blinks, and the ocular surface remains protected and comfortable. The goal of developing metrics such as OPI is to allow for a precise, reproducible measure of dry eye that might be modifiable with treatment. While there are many factors contributing to the dry-eye phenotype (such as age, environment, medications and visual activity), all of these converge on the tear film and its primary homeostatic regulator, the blink.
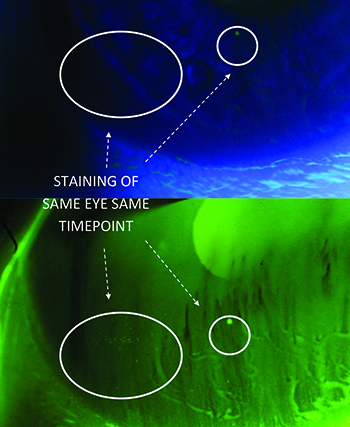
Our attention to staining as a precise endpoint resulted in using a yellow low-pass filter at the slit lamp. This filter bumps up the scoring of SPK by a whole grade, a heightened sensitivity that has multiple implications, both to the practicing clinician and to the translational researcher grading dry-eye subjects. Firstly, normals might all have staining if you look close enough; secondly, the filter can provide a tipping point for both false positive and false negative diagnoses of dry eye; and thirdly, total clearing of staining is not physiological. We have continued to improve on staining assessments by adding automated analysis of images for systematic grading of punctate keratitis on a numerical scale, and our article on the subject has been accepted for publication in a 2015 issue of Investigative Ophthalmology and Visual Science. This system provides an extremely consistent output of data, particularly across multi-centered studies that might involve as many as 30 sites.
Automating Blink Analysis
Our most recent work on blink telemetry involves automated, continuous monitoring of the blink throughout the day, to further dissect patterns of blink behavior in normal and dry-eye subjects.11-13 One observation from these studies is that dry-eye patients have prolonged blink closure times, more than six times longer than normal subjects, a phenomenon similar to the micro-sleeps described in the literature on fatigue.13 These extended blinks result in dry-eye subjects having their eyes closed five times longer per minute than a normal subject.11 A number of reports have shown that the closed-eye tear film has a higher relative amount of inflammatory cells and IgA, suggesting a cleanup or surface maintenance function during sleep.14,15 It’s possible that these subconscious closures are a related compensatory mechanism.
|
One of our first efforts in evaluating therapeutics with the CAE investigated the activity of a topical formulation of ecabat sodium. This compound had no effect except that it normalized blink rate, reducing it by half. This finding led us to the realization that drugs with potential for treating dry eye would have to positively affect blink rate, and that this was a sensitive indicator of therapeutic benefit, as well as an early protective mechanism. Furthermore, halving blink rate seemed significant to visual function and conversely, doubling blink rate to maintain tear-film stability, seemed to be the catch-22 linking blink to fatigue, and brought us back to the literature on fatigue, alertness and blink as an indicator of both.16
Other more subtle and realistic assessments of visual function are needed to understand how dry eye is affecting the life of the patient. We have recently been analyzing this through the study of reading—both short and long passages as well as reading silently and out loud—in an effort to move this qualitative measure forward into clinical trials as a quantitative, real-time measure of one of the most important patient activities. We also study reading rates in the context of blinking, and have found that, in studying either blink or reading, the crucial element is an understanding of the physiology that allows us to devise accurate, reproducible metrics that yield clinically meaningful measures.
Assessment of symptoms is of critical importance and highly difficult due to their subjective nature. We discovered while carrying out blink telemetry studies that dry-eye subjects have lower blink rates during times of fewer symptoms, suggesting that blink data might be a useful surrogate to corroborate dry-eye symptom measures. Thus, blink links not only to tear-film stability but also to symptoms, and could provide corroboration for the often less-than-reliable data collected from subjects in diaries. Nevertheless, validating diary data in a clinical trial of highly symptomatic diseases such as allergy and dry eye remains critical, and we have been working with IT to produce cell phone app diaries as well as hardware that allow subjects to monitor and image ocular redness and symptoms on their own in real time. These images are uploaded to an automatic system of redness grading that has been shown to highly agree with clinical grading.17 Automation is the future of clinical trials, and we’re working hard to develop software systems that automate grading of redness as we did staining, to minimize investigator subjectivity and tighten the dataset.
The CAE has proven invaluable in our understanding of dry eye. The array of exogenous and endogenous variables that impact dry eye partly explains why development of effective dry-eye therapies has been so problematic. By exposing dry-eye subjects to a hot, dry and ventilated environment, and providing them with a continuous visual task, baseline conditions of dry eye are exacerbated in equal measure in the entire cohort. This model constructs a stable, heightened baseline for a population that otherwise would be highly variable in their natural state. From this platform, the effect of modifications in environment or addition of a therapeutic is more readily visible.10,18,19
We use the CAE model to inform our study designs and refine our understanding of disease subtypes. Screening with the CAE was used to identify modifiable patients in Phase III studies of MIM-D3 and Lifitegrast,20,21 and served to help establish the relationship between dry eye and allergy.22 Models such as the CAE can be invaluable tools to actually identify which patients might respond to a specific therapeutic: subgroups that might be more sensitive to a mucogenic, an anti-inflammatory or a tear substitute tailored to relieve symptoms. We have used the CAE to test contact lens tolerability, a practical outcome that provides information for both patient and clinician. The CAE has also been used to aid in the development of tear substitutes such as Systane (Alcon Laboratories).
It is readily apparent from this example of a seemingly arcane physiological phenomenon such as blink that one clinical observation can lead to a generation of translational research. From the clinic to the lab, the knowledge gained from the physiology of blink has led to numerous byroads that young researchers might follow. Likewise, when new compounds are conceived, it is translational exploration that brings these breakthrough discoveries from test tube to treatment. Allowing for this progress are advances in translational research like those we’ve discussed here, as well as those we’ve left for later columns, such as drug formulation and safety issues.
Translational medicine may streamline the plodding stepwise process typical of therapeutic development, but in the end all must face the gauntlet of clinical assessment, and we’re all part of the translating process. REVIEW
Dr. Abelson is a trustee of the Schepens Eye Research Institute, emeritus surgeon of the Massachusetts Eye and Ear Infirmary, and a clinical professor of ophthalmology at Harvard Medical School. Ms. Smith and Dr. McLaughlin are medical writers at Ora Inc.
1. Duda GN, Grainger DW, Frisk ML, et al. Changing the mindset in life sciences toward translation: A consensus. Sci Transl Med 2014;6:264:264cm12.
2. Edelman ER, LaMarco K. Clinician-investigators as translational bioscientists. Sci Transl Med 2012;4:135:135fs14.
3. Kurplinski K, Johnson T, Kumar S, et al. Mastering translational medicine. Sci Transl Med 2014;6:218:218fs2.
4. Abelson MB, Holly FJ. A tentative mechanism for inferior punctate keratopathy. Am J Ophthalmol 1977;83:6:866-9.
5. Abelson MB, Ousler GW 3rd, Nally LA, et al. Alternative reference values for tear film break up time in normal and dry eye populations. Adv Exp Med Biol 2002;506(B):1121-5.
6. Ousler GW 3rd, Abelson MB, Nally LA, et al. Evaluation of the time to “natural compensation” in normal and dry eye subject populations during exposure to a controlled adverse environment. Adv Exp Med Biol 2002;506(B):1057-63.
7. The International Dry-Eye Workshop. Methodologies to diagnose and monitor dry eye disease: Report of the diagnostic methodology subcommittee of the international dry eye workshop. Ocul Surf 2007;5:2:108-152.
8. Ousler GW 3rd, Hagberg KW, Schindelar M, et al. The Ocular Protection Index. Cornea 2008;27:5:509-13.
9. Walker PM1, Lane KJ, Ousler GW 3rd, Abelson MB. Diurnal variation of visual function and the signs and symptoms of dry eye. Cornea 2010;29:6:607-12.
10. Ousler GW, Gomes PJ, Welch D, Abelson MB. Methodologies for the study of ocular surface disease. Ocul Surf 2005;3:3:143.
11. Ousler GW 3rd, Abelson MB, Johnston PR, Rodriguez J, Lane K, Smith LM. Blink patterns and lid-contact times in dry-eye and normal subjects. Clin Ophthalmol 2014;5:8:869-74.
12. Johnston PR, Rodriguez J, et al. The interblink interval in normal and dry eye subjects. Clin Ophthalmol 2013;7:253-9.
13. Rodriguez JD, Ousler GW 3rd, Johnston PR, et al. Investigation of extended blinks and interblink intervals in subjects with and without dry eye. Clin Ophthalmol 2013;7:337.
14.Bitton E, Keech A, Jones L, Simpson T. Subjective and objective variation of the tear film pre- and post-sleep. Optom Vis Sci 2008;85:8:740-9.
15. Sack RA, Beaton A, Sathe S, et al. Towards a closed eye model of the pre-ocular tear layer. Prog in Retin Eye Res 2000;19:649.
16. Schleicher R, Galley N, Briest S. Blinks and saccades as indicators of fatigue in sleepiness warnings: Looking tired? Ergonomics 2008;51:7:982-1010.
17. Rodriguez JD, Johnston PR, Ousler GW 3rd, et al. Automated grading system for evaluation of ocular redness associated with dry eye. Clin Opthalmol 2013;7:1197.
18. Abelson R, Lane KJ, Rodriguez J, et al. A single-center study evaluating the effect of the controlled adverse environment (CAE) model on tear film stability. Clin Ophthalmol 2012;6:1865-72.
19. González-García MJ, González-Sáiz A, de la Fuente B, et al. Exposure to a controlled adverse environment impairs the ocular surface of subjects with minimally symptomatic dry eye. Invest Ophthalmol Vis Sci 2007;48:9:4026-32.
20. Meerovitch K, Torkildsen G, Lonsdale J, et al. Safety and efficacy of MIM-D3 ophthalmic solutions in a randomized, placebo-controlled Phase 2 clinical trial in patients with dry eye. Clin Ophthalmol 2013;7:1275-85.
21. Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: Results of the OPUS-1 phase 3 study. Ophthalmology 2014;121:2:475.
22. Gomes PJ, Ousler GW, Welch DL, et al. Exacerbation of signs and symptoms of allergic conjunctivitis by a controlled adverse environment challenge. Clin Ophthalmol 2013;7:157-65.