Managing patients with diabetic retinopathy can be a challenge. Not only do some patients remain asymptomatic and unaware of the advancing disease, many are not even going for regular eye exams. Diabetic retinopathy is the number one cause of blindness in American adults ages 20 to 74, and although it’s recommended that diabetics receive annual eye exams, more than 50 percent of those with DR don’t receive necessary screening.1
Understanding this patient population is integral to treating their disease, and that includes comorbidities and risk factors that could contribute to their outcome. We spoke with several retina specialists about their standards of care for proliferative diabetic retinopathy specifically, how they determine if a patient is best suited for anti-VEGF or panretinal photocoagulation and what they suggest to improve patient trust and follow up.
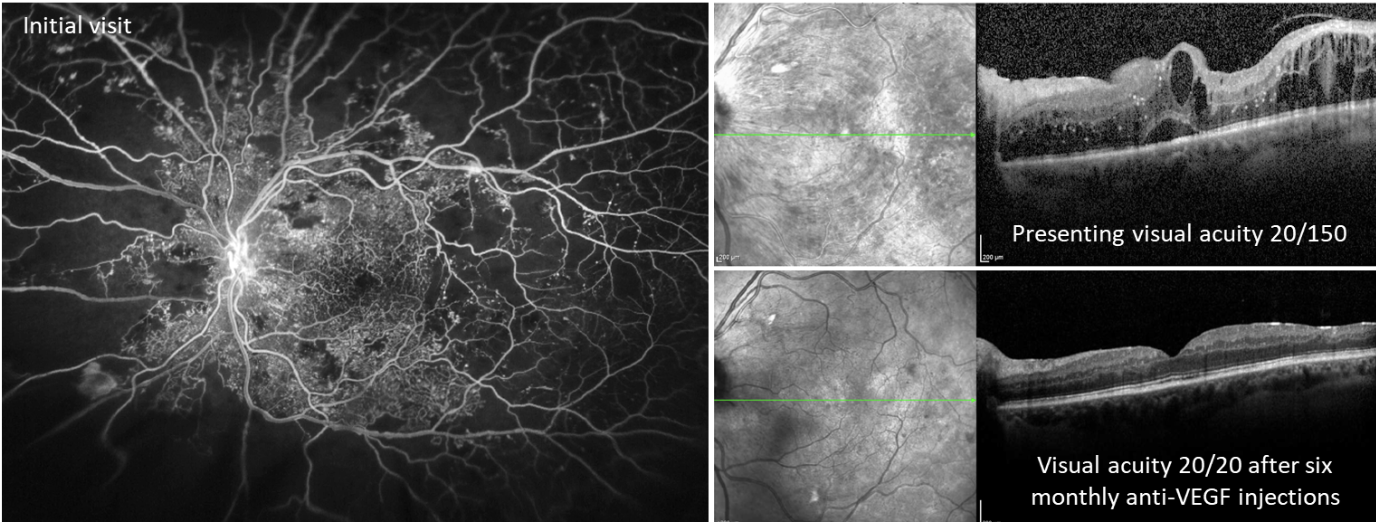 |
| Figure 1. Images from a male patient in his 30s with poorly controlled type 2 diabetes (hemoglobin A1c >12%) and no prior eye care. Fluorescein angiography on initial visit showed extensive areas of peripheral non-perfusion with fronds of neovascularization (left eye shown). OCT showed severe center-involving diabetic macular edema (top right) which responded well to intravitreal anti-VEGF (aflibercept) injections (bottom right). By one year after presentation, he was 20/20 in both eyes with an A1c <7% due to improved adherence to diabetes care and better systemic glucose control. Photo: Ian C. Han, MD. |
PDR Symptoms and Screening
Proliferative diabetic retinopathy distinguishes itself from non-proliferative diabetic retinopathy in its severity. PDR’s tell-tale sign is neovascularization, brought on by poor glycemic control, high blood pressure, high cholesterol and other chronic health issues. When left untreated, the risk for retinal detachment increases.
Vascular damage often occurs more in the peripheral areas of the retina, says Jason Hsu, MD, co-director of retina research at Wills Eye Hospital, assistant professor of clinical ophthalmology at Thomas Jefferson University Hospital in Philadelphia, and a managing partner of Mid Atlantic Retina.
“These patients may go to their general eye doctor with little or no symptoms or maybe just for a pair of glasses and are incidentally found to have massive neovascularization. Their eye doctor may say, ‘Wow, you have a lot of damage’ or ‘You’re at high risk of losing your vision,’ and it’s not uncommon that they don’t believe the doctor because their vision is still pretty good,” he says. “If they do present with symptoms, they may include new onset of floaters from some vitreous hemorrhage. Those are very common scenarios for how these patients are first diagnosed. Another thing that can occur in all diabetics where there’s proliferative or non-proliferative diabetic retinopathy is diabetic macular edema, and that would cause some more central blurring and could be more symptomatic as well.”
Diagnosing asymptomatic patients may take some careful screening, advises Ian C. Han, MD, an associate professor in the department of ophthalmology and visual sciences at the University of Iowa Hospital and Clinics.
“For me, screening still starts with listening to the patient and obtaining a careful history,” Dr. Han says. “For example, if you have somebody who’s just coming in for a new eye examination, but they’ve had a diabetes diagnosis for 15 years, your initial examination is already alert to the strong possibility of PDR. I often tell the residents and fellows to default to the assumption that the patient has PDR—and prove to yourself on clinical examination that they don’t—otherwise you might miss signs of the disease.”
Different imaging modalities include baseline fundus photography and OCT, as well as fluorescein angiography and OCT angiography. “PDR is kind of tricky because, on an OCT, you might not see a whole lot,” Dr. Hsu says.
Dr. Han agrees. “PDR patients actually have a pretty bland-appearing fundus; you may not see a ton of hemorrhages and such. Someone in a routine eye clinic may see a patient with 15 to 20 years of poorly controlled diabetes and see a dot hemorrhage here or there and assume that they only have minimal disease because blood is the most apparent fundus finding when in fact, it’s a PDR with neovascularization that is missed,” he says.
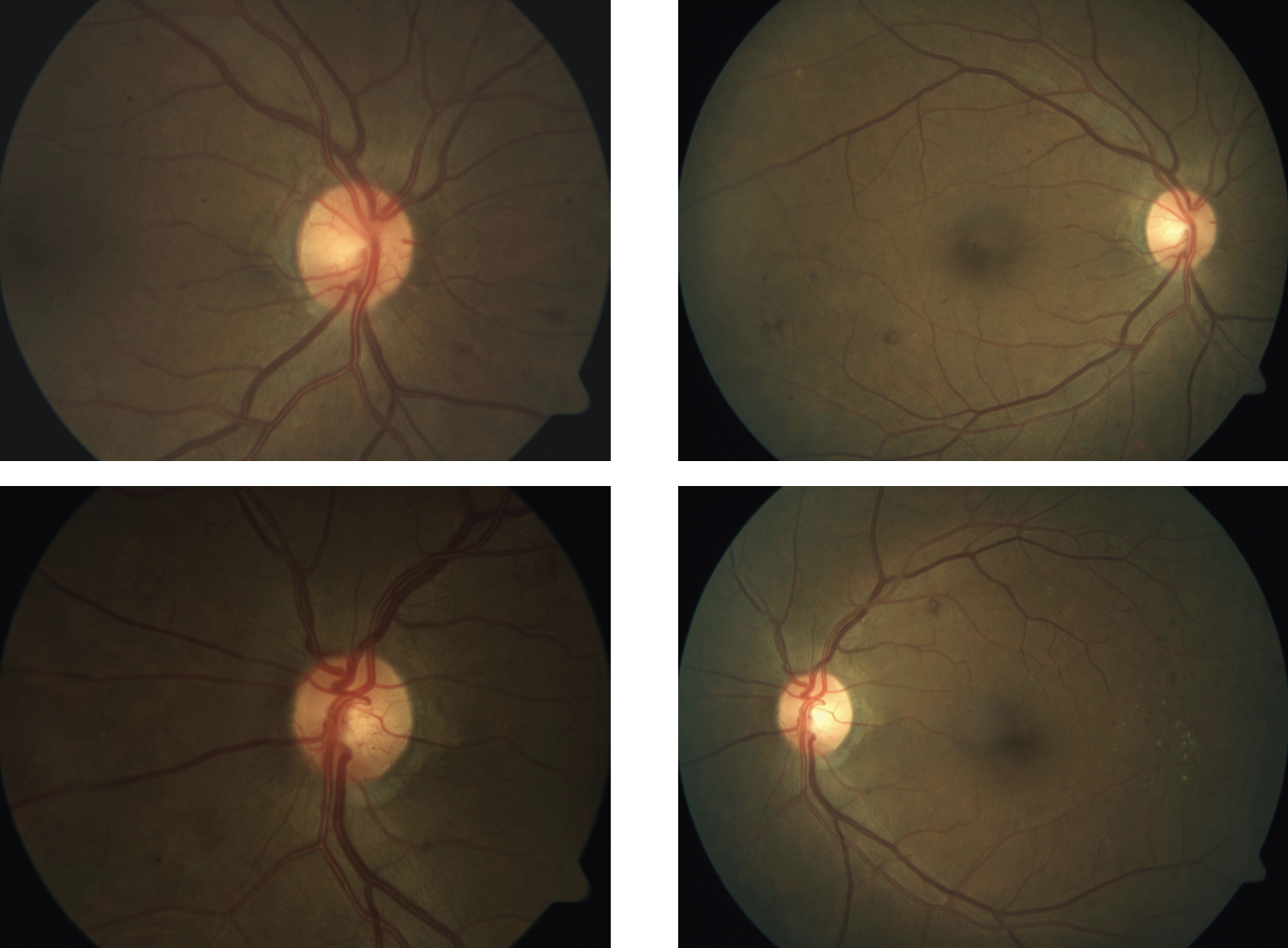 |
| Figure 2. This patient presented with PDR in both eyes, shown in the preop fundus images taken in April 2017 (left column). After treatment with an anti-VEGF, the PDR is no longer apparent in fundus images one month later (right column). Photo: Jennifer I. Lim, MD. |
“Now, if you’re getting a wider field OCT, then you can sometimes pick up neovascularization along some of the arcades and over the optic nerve,” Dr. Hsu says. “The neovascularization often forms hyperreflective membranes, almost like an epiretinal membrane but often with greater separation above the plane of the retina or the optic nerve. One thing that’s helpful to pay attention to is the near infrared image of the macula that’s used to correlate where the OCT cut is going through. Neovascularization on the near infrared image usually looks dark and obscures part of the vascular arcades or the optic nerve. While that’s not 100 percent proof that it’s neovascularization, it can clue you in and tell you to look more carefully.”
“With ischemia and vascular remodeling, intraretinal hemorrhages may be less prominent, so ophthalmologists may miss some of the more severe or more advanced findings that fall in the category of PDR unless you study the retinal blood vessels carefully on exam or imaging,” Dr. Han says, with a recommendation not to over-rely on technology to make the diagnosis. “Clinical context is still really important. Even with modern technology, some subtle vascular abnormalities may not be as apparent unless you go looking for them or they’re below the resolution or the quality of your image,” he says.
Treatment Decisions and Debates
Treating PDR depends on its severity. “Laser is the ‘traditional’ therapy that has been around for decades,” says Dr. Hsu. “In this situation, we do panretinal photocoagulation. We create a pattern of laser that is spaced out by about one spot width and typically staying at least 1 to 2 disc diameters away from the major arcades and the optic nerve, so as to not interfere quite as much with the patient’s perception of their peripheral vision.” A 1976 study was the first to show PRP’s benefits in lowering the risk of vitreous hemorrhage and reducing the risk of vision loss by 50 percent.2
“I definitely try to start with PRP first when I can, but the problem is, being in a retina referral practice, the patients are coming in because they have vitreous hemorrhage. When there’s vitreous hemorrhage present, the laser isn’t effective because the blood in the vitreous scatters the laser beam, so you can’t get good uptake,” says Dr. Hsu. “In earlier stages, treatment’s a little more of a clinician judgment call, depending on whether they feel the patient is going to follow up regularly. Sometimes you can see people with some peripheral neovascularization but no vitreous hemorrhage and no symptoms. You don’t have to treat those eyes according to the studies because they may still have good outcomes if you wait and only treat when high-risk characteristics develop.”
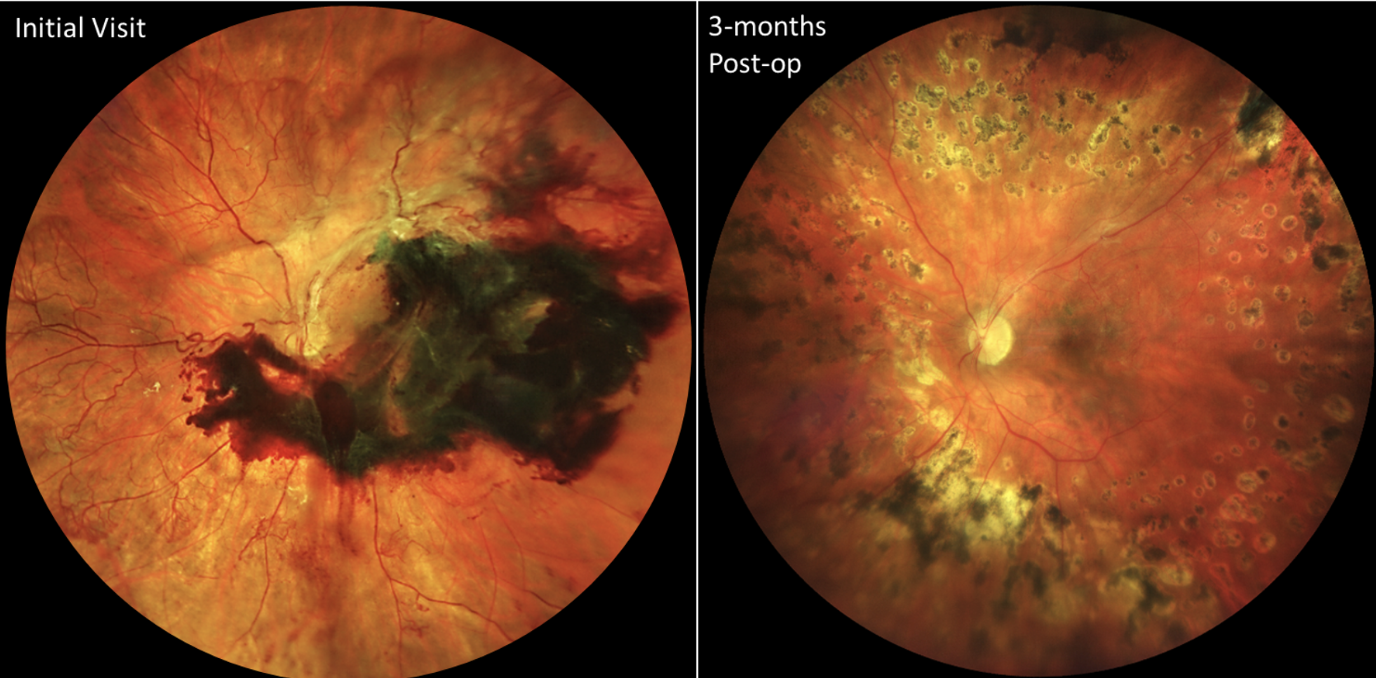 |
| Figure 3. These images are from a female patient in her 30s with poorly controlled type 1 diabetes. Due to social determinants of health (e.g., limited transportation), she had no prior eye care, and her right eye went blind due to neovascular glaucoma. She presented when the better-seeing left eye declined to count fingers visual acuity due to a subhyaloid hemorrhage and tractional retinal detachment with extensive, mature-appearing neovascularization of the disc and elsewhere (left). After prompt vitrectomy with membrane removal and laser placement, she recovered to 20/25 visual acuity (right), which she has maintained over years of subsequent follow up. Photo: Ian C. Han, MD. |
There are well-known side effects to note with PRP, including decreased contrast sensitivity,3 loss of visual acuity and constriction of the peripheral visual field.4
“One of the downsides of laser, which I discuss with patients, is that it may decrease peripheral vision and even night vision,” says Dr. Hsu. “That’s been well-demonstrated because you’re essentially sacrificing the peripheral retina to save the central retina. But interestingly enough, I would say it’s very rare for patients to actually complain after the laser that they lost peripheral vision because a lot of their peripheral retina is already pretty ischemic and not working too well to begin with. I’m also not doing super heavy PRP, like what may have been done decades ago before anti-VEGF was available. I still have some patients who had laser done 30 to 40 years ago who are doing great with 20/20 vision but the laser goes all the way to just outside the arcades. I assume that they have a much smaller field of vision, but they don’t complain about it because they’re still seeing well.”
The other treatment option is anti-VEGF therapy. “Anti-VEGF blocks the major pathway that’s leading to the neovascular growth we see in PDR and it’s quite amazing,” says Dr. Hsu. “For example, if you do an injection of an anti-VEGF agent and bring the patient back a few hours later or the next day, the vessels often just sort of melt away really quickly in response. It’s also great that it’s not destructive like PRP.”
“When I started in ophthalmology, anti-VEGF was still fairly new in routine clinical practice, and we didn’t expect patients to turn around on the one-way train of disease progression in PDR. All you were hoping for was to halt the progression of disease,” says Dr. Han. “The initial indication for anti-VEGF therapy wasn’t for PDR, but for diabetic macular edema. For example, the RISE and RIDE clinical trials with ranibizumab investigated the effect of anti-VEGF therapy in DME, but amazingly, it also reversed diabetic retinopathy severity for the majority of patients. Now, anti-VEGF therapy has a strong track record of working well and being effective, with the literature to support multiple anti-VEGF agents (bevacizumab [Avastin], ranibizumab [Lucentis], aflibercept [Eylea], etc.) with other newer drugs also now available (faricimab [Vabysmo]).”
The CLARITY study, published in 2017, compared one-year best corrected visual acuity letter change from baseline results of PDR patients treated with the anti-VEGF aflibercept vs. PRP.5 At 52 weeks, the outcome showed aflibercept was non-inferior and superior to PRP (mean best corrected visual acuity difference 3.9 letters [95% CI 2.3–5.6], p<0.0001). This backed up earlier findings of the Diabetic Retinopathy Clinical Research Network’s Protocol S trial in 2015, which showed ranibizumab treatment resulted in visual acuity that was non-inferior to PRP when measured through two years.6
“That was a landmark study that really led to this paradigm shift of doing anti-VEGF over PRP because it shows equivalent outcomes with anti-VEGF therapy compared to PRP,” says Dr. Hsu.
“In recent years, treatment for PDR has shifted toward injections, certainly if there’s DME, and then you’ll find a wide variety of practice patterns from PDR with no DME or with just neovascular complications,” says Dr. Han. “I think that has to do with what you think is the most effective treatment for the degree or severity of neovascularization that’s out there. ‘High-risk’ PDR was defined decades ago (in the Diabetic Retinopathy Study) before anti-VEGF therapy or modern vitrectomy surgery. Nowadays, I think of a patient as being ‘high-risk’ based on social determinants of health that impact whether the patient can stick with a treatment regimen, or aspects of their disease that may cause irreversible vision loss such as traction leading to retinal detachment, or neovascular glaucoma.”
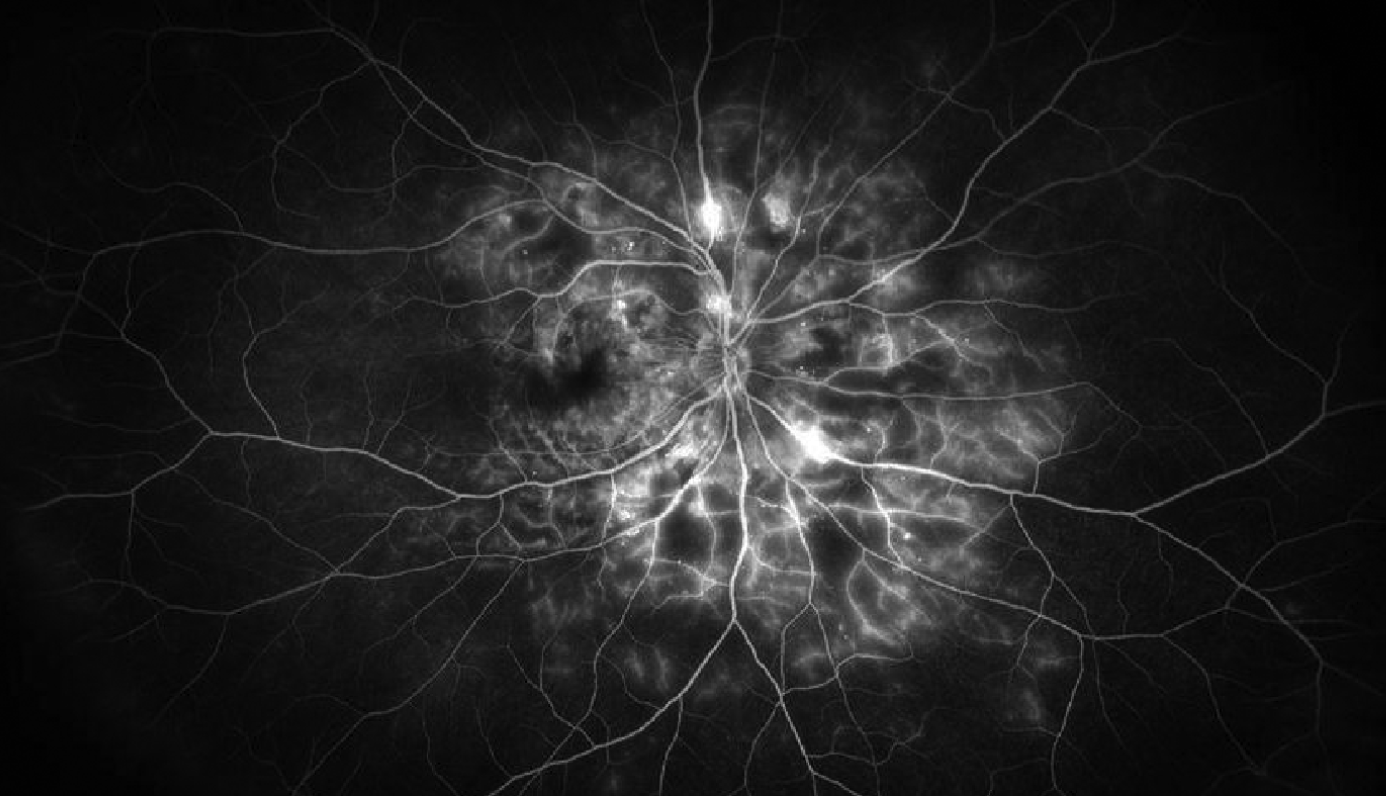 |
| Figure 4. Wide-field fluorescein angiography image of the right eye of a patient with proliferative diabetic retinopathy demonstrating hyperfluorescent areas of vascular staining and leakage along with hypofluorescent areas of capillary nonperfusion. Photo: Jason Hsu, MD. |
The fact that anti-VEGF works so well can be to the patient’s detriment, Dr. Hsu adds. “Sometimes it works so well that patients may think ‘Oh, you cured me. I don’t need to come back.’ And they will see really well until the next bleed or something else happens,” he says.
This is the crux of an ongoing debate among retina specialists. Both anti-VEGF and PRP require regular follow up, and considering anti-VEGF’s lack of durability, some in the field argue it’s not strong enough as a solo treatment.
“We see debates at meetings where there’s a back and forth over which treatment is better, but I do agree the Holy Grail would be to come up with a non-destructive treatment,” says Dr. Hsu. “Some have latched on to anti-VEGF as the non-destructive treatment, because the idea is that, as retina specialists, we want to preserve the functioning of the retina and not destroy it. And the concept of sacrificing the peripheral retina to save the central retina is not necessarily appealing to a lot of people.
“But I think on the flip side, we’re just not there yet,” Dr. Hsu continues. “The anti-VEGF era is here, but the delivery of it isn’t ideal for these patients. Looking back at Protocol S, they went out to five years and they found that with the anti-VEGF injection, patients still needed on average three injections a year. So it’s not like there’s a long-term cure with continuous anti-VEGF therapy—as far as we know, they need to keep getting it indefinitely. If there was a one-and-done treatment that would provide a long-term anti-VEGF blockade, maybe that would be the Holy Grail and would be enough to move the needle away from laser in my mind. But personally, I think we’re not there yet, because there’s just too much at stake for these patients not to have PRP.”
There’s also some benefit to combining treatments. A 2022 collective review of studies assessing the impact of PRP and anti-VEGF for diabetic retinopathy found nine trials showing a combination therapy has a better impact on improving or delaying vision deterioration in BCVA, compared to monotherapy, as well as improving neovascularization regression with no potential increased incidence of adverse events.8
“My message for specialists who administer anti-VEGF injections is that it’s critical to continue to perform PRP in eyes with PDR,” says Dr. Hsu. “I realize that our practices are now built around injections, and it throws a wrench in the patient flow by throwing in a laser. PRP takes more time, but we shouldn’t be sacrificing laser because it’s inconvenient.”
Before beginning any treatment, retina specialists have to ask themselves: How likely is it that this patient will follow through with treatment?
“Risk of loss to follow-up is huge,” says Jennifer I. Lim, MD, the Marion H. Schenk Esq., chair in ophthalmology for research in the aging eye, a University of Illinois at Chicago distinguished professor of ophthalmology, the vice chair for diversity and inclusion, and director of the retina service at UIC. “If a patient is being given an anti-VEGF and they don’t follow up, the downside is so much worse than if they were given PRP. If they have one anti-VEGF injection and they don’t show up again, I have no idea: Did it work? Did it regress in a month, three months? And so those patients aren’t the type I would want to put on an anti-VEGF.”
She says some risk factors of losing patients to follow-up include being younger in age, working jobs where there aren’t opportunities to take time off for appointments, if patients have to travel long distances for care, as well as their socioeconomic status and race.
Dr. Hsu studied just this7 and identified three key risk factors of loss to follow-up (LTFU): type of procedure; age; and race. “LTFU is a bigger problem than we thought,” he says. “Our research fellow, Anthony Obeid, and I first published the paper on this back in 2018. We were just looking at our own practice and focusing on high-risk patients with PDR who had either injections or PRP. The question we asked was, when a patient gets an anti-VEGF injection or PRP, how many of them don’t come back for at least a year or more immediately after that treatment? We found that about a quarter of these patients in our practice were lost to follow-up and might not come back for a year or more—if ever.
“It’s really eye-opening and scary,” Dr. Hsu adds. “In our study, some of the risk factors for being lost to follow-up seemed to be younger age and being African American or Hispanic. We also conducted a zip code analysis of where the patients live and looked at the average adjusted gross income in that zip code in order to get an idea of income level. Patients who lived in areas with a lower AGI had a higher risk of loss to follow-up.”
This then prompted another study that evaluated the outcomes of eyes that were LTFU for more than six months after their procedure. VA worsened significantly in both groups, 20/187 for anti-VEGF and 20/83 for PRP, but the PRP group returned to baseline after additional therapy, yet the anti-VEGF group didn’t have as much improvement at 20/166.9 The authors also discovered that 17 percent of eyes in the anti-VEGF group developed a tractional retinal detachment at the return visit, which increased to 30 percent by the final visit. In the PRP group, no eyes had a TRD at the return visit and only 2 percent had a TRD by the final visit.9
For this reason, some may even consider going with PRP in the first place. “With anti-VEGF, I never feel comfortable saying, ‘Okay, I can see you back in a year,’ ” says Dr. Lim. “I’ve had patients who are very motivated to come in for their treatments, and even then they sometimes break in the middle, so lately I’ve been finding myself thinking it would be easier for the patient—and me in some ways—to just do the PRP because I think it’s a more permanent solution. And you worry less about loss to follow-up, because there are some things out of the patient’s control.”
Dr. Hsu says there’s no formula for predicting who will be lost to follow-up. “Many may think they can predict who will follow up by looking at certain patient characteristics,” he says. “Unfortunately, we don’t know when someone may lose their job or have an illness or some other issue and be unable to return. Still, we’ve got to encourage follow-up and keep track of these patients. It really is worthwhile to have someone on your staff tracking every patient who receives an injection or laser. If they’re not coming back, bug them like crazy with phone calls, certified letters, whatever it takes. We’re submitting a study soon showing our results after hiring a full-time person whose primary job is to do just that. While we found it made a difference, it still wasn’t 100 percent.”
Building Patient Trust
PDR treatment will be most successful when the physician-patient relationship is strong, say these doctors.
“Whenever I’m approaching treatment for PDR, I sit the patient down and very carefully explain to them what the consequences are if they don’t show up,” says Dr. Lim. “I develop a relationship similar to a team effort. Their job is to show up and report symptoms, and my job is to make sure that I treat them appropriately. I also encourage buy-in from them on what treatment we do, so it’s a shared decision. Then patients feel like they’ve had a say in things and it wasn’t being dictated to them.”
Managing PDR for patients may also end up turning a patient’s whole health in a positive direction, suggests Dr. Han. “With diabetic retinopathy, patients have seen a lot of medical providers in their lives already and heard a lot of bad news. Sometimes the thing that wakes them up is the seriousness of their eye condition, and it does take a lot to build trust. Treatment is about trust. This is a systemic disease, not just ocular, but you can save a life and turn the whole body—the whole patient—around. The eyes are often valued most and can be the motivation to get their life back in balance.”
Dr. Han reports no disclosures. Dr. Hsu is a consultant for IvericBio, Gyroscope Therapeutics and Bausch + Lomb, and receives grant support from Genentech/Roche, IvericBio and Aldeyra Therapeutics. Dr. Lim is a consultant for Alcon, Aldeyra Therapeutics, Allergan, Chengdu Kanghong, Eyenuk, Genentech, IvericBio, Novartis, Regeneron and Santen.
1. Fathy C, Patel S, Sternberg P Jr, Kohanim S. Disparities in adherence to screening guidelines for diabetic retinopathy in the United States: A comprehensive review and guide for future directions. Semin Ophthalmol 2016;31:4:364-77.
2. Preliminary report on effects of photocoagulation therapy. The Diabetic Retinopathy Study Research Group. Am J Ophthalmol 1976;81:4:383-96.
3. Preti RC, Ramirez LM, Monteiro ML, Carra MK, Pelayes DE, Takahashi WY. Contrast sensitivity evaluation in high risk proliferative diabetic retinopathy treated with panretinal photocoagulation associated or not with intravitreal bevacizumab injections: A randomised clinical trial. Br J Ophthalmol 2013;97:7:885-9.
4. Photocoagulation treatment of proliferative diabetic retinopathy: The second report of diabetic retinopathy study findings. Ophthalmology 1978;85:1:82-106.
5. Sivaprasad S, Prevost AT, Vasconcelos JC, Riddell A, Murphy C, Kelly J, Bainbridge J, Tudor-Edwards R, Hopkins D, Hykin P; CLARITY Study Group. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): A multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet 2017;3:389:10085:2193-2203.
6. Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, Baker CW, Berger BB, Bressler NM, Browning D, Elman MJ, Ferris FL 3rd, Friedman SM, Marcus DM, Melia M, Stockdale CR, Sun JK, Beck RW. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA 2015;314:20:2137-2146.
7. Obeid A, Gao X, Ali FS, Talcott KE, Aderman CM, Hyman L, Ho AC, Hsu J. Loss to follow-up in patients with proliferative diabetic retinopathy after panretinal photocoagulation or intravitreal anti-VEGF injections. Ophthalmology 2018;125:1386-1392.
8. Zhang W, Geng J, Sang A. Effectiveness of panretinal photocoagulation plus intravitreal anti-vegf treatment against prp alone for diabetic retinopathy: A systematic review with meta-analysis. Front Endocrinol (Lausanne) 2022;29:13:807687.
9. Obeid A, Su D, Patel SN, Uhr JH, Borkar D, Gao X, Fineman MS, Regillo CD, Maguire JI, Garg SJ, Hsu J. Outcomes of eyes lost to follow-up with proliferative diabetic retinopathy that received panretinal photocoagulation versus intravitreal anti-vascular endothelial growth factor. Ophthalmology 2019;126:3:407-413.



