Encountering dry-eye signs and symptoms in glaucoma patients is fairly common. Chronic use of IOP-lowering medications that contain preservatives is often the culprit, coupled with the fact that both diseases tend to affect an older population.
“It puts patients in a difficult position because they rely on these medications to control their glaucoma, but those same medications may be causing or exacerbating their ocular surface disease,” says Sarwat Salim, MD, FACS, a professor of ophthalmology and director of the Glaucoma Service at the New England Eye Center at Tufts University School of Medicine in Boston. “Considering the effect OSD and glaucoma have on quality of life, it’s important to take steps to reduce preservative exposure.”
In this article, experts discuss some of the medical, procedural and surgical options for managing glaucoma-related OSD.
OSD Testing
Devising a management strategy starts with recognizing that your patient has OSD. Dr. Salim says this step is critical but often challenging because the signs and symptoms of OSD don’t always align. “An asymptomatic patient might have severe surface staining and MGD, and a symptomatic patient might have only minimal surface staining,” she says. “The level of comfort or discomfort patients feel isn’t always a reliable means of assessing OSD. Incorporating OSD testing, such as TBUT, surface staining and Schirmer’s test in your management of glaucoma patients can help you identify who needs treatment and which dry-eye mechanism is behind it.
“MMP-9 and osmolarity testing are also particularly useful,” she continues. “One can use InflammaDry (Quidel) to evaluate MMP-9 levels in the tear film. It’s common for glaucoma patients on preserved medications to exhibit elevated MMP-9 levels; this indicates an inflammatory etiology. In such cases, consider reducing the number of medications with preservatives or starting anti-inflammatory treatment.”
To assess a patient’s level of evaporative dry eye, she performs osmolarity testing. “Osmolarity is a balance of evaporation, drainage and tear production,” she says. “It can be tested with a handheld osmometer (TearLab) to assess the level of evaporative dry eye. When patients have hyperosmolarity, their tears evaporate too quickly. We often see this in patients on topical glaucoma medications.” The TearLab osmolarity system defines abnormal osmolarity as >300 mOsm/L or when the inter-eye difference is >8 mOsm/L.
“Additionally, ocular surface interferometry and infrared meibography (LipiView, Johnson & Johnson Vision) can help you assess, respectively, the tear film lipid layer and meibomian gland structure,” she notes. “These data will help you determine the type of treatment your patient needs.” Dr. Salim recommends performing dry-eye tests at baseline and at regular intervals to monitor the impact of treatment interventions.
Reducing Preservatives
 |
| Chronic use of topical, preservative-containing glaucoma medications can cause or exacerbate ocular surface disease. The literature also suggests an association between the number of drops a patient takes and their compliance with medication: The more complex the regimen, the lower the compliance, which may result in glaucoma progression. Fixed-dose combination drops may help address this, while limiting preservative exposure. |
Preservatives play an important antimicrobial role in multidose ophthalmic medications, but they may also cause irritation, redness and overall discomfort, particularly with concurrent MGD or blepharitis. Benzalkonium chloride, the most widely used preservative, is toxic to the cornea, yet it also improves the effectiveness of some medications by disrupting the junctions of the epithelium to facilitate drug penetration into the eye, according to Thomas E. Bournias, MD, a glaucoma specialist, the director of the Northwestern Ophthalmic Institute and an assistant professor of clinical ophthalmology at Northwestern University School of Medicine. “It has an additive effect on the corneal surface, so the higher the concentration and/or the more BAK-containing medications a patient is on, the greater the effect,” he says.
Dr. Salim says that expanding the number of preservative-free glaucoma medication options and making them affordable and accessible would help to reduce the burden of OSD in glaucoma. “We know that switching to preservative-free formulations leads to improvement in dry eye,” she says. “Currently, however, it’s not always possible to switch a patient’s glaucoma drops to preservative-free formulas. Availability is one issue, and cost is another.”
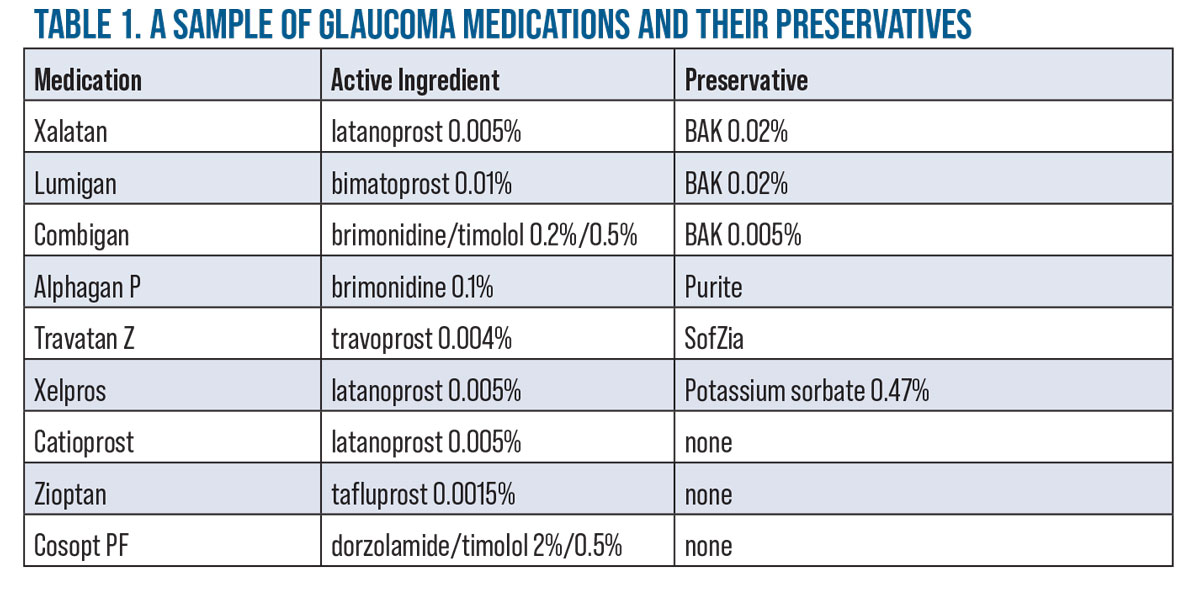
“If you can’t avoid BAK, using a lower amount can help,” says Dr. Bournias. “Some patients can tolerate drops with lower concentrations of BAK such as Xalatan (BAK 0.02%, Pfizer) or Combigan (BAK 0.005%, Alcon).” (For more BAK alternatives, see table below.) Dr. Bournias adds that he’s also used Xelpros (Sun Pharma), a non-BAK latanoprost and Catioprost (Novagali), an unpreserved latanoprost-cationic emulsion, for OSD patients.
An additional benefit of preservative-free medication is the single-dose vials they come in, experts say. “Many patients enjoy the convenience of having a vial or two of drops for the day,” Dr. Bournias says. “It’s difficult for patients to refill their multidose bottles if they run out too soon (e.g., in the event they squeeze out too much) but the individual vials tend to last them the whole month.”
“One other option to decrease preservative exposure is to convert your patient’s glaucoma medications to fixed-dose combination products,” says Dr. Salim. “Compounding pharmacies have affordable fixed-dose combination formulations of preservative-free glaucoma medications such as dorzolamide/timolol and brimonidine/timolol.”
She says that combination products have the added benefit of greater simplicity for the patient, leading to increased compliance with fewer drops to instill. “Combination drops also help to avoid the washout effect, which often happens when patients quickly instill multiple medications, one after the other. However, you aren’t able alter the concentrations of the individual medications or the time of day they’re administered when the drops are combined.”
Christopher J. Rapuano, MD, chief of the Wills Eye Cornea Service and a professor of ophthalmology at Sidney Kimmel Medical College of Thomas Jefferson University in Philadelphia, notes that there are a number of systemic medications that can cause dry eye, so it’s important to ask your patients what other medications they’re taking. “Antihistamines such as Benadryl dry out the sinuses, which is great for some sinus conditions, but it also dries out the eyes,” he explains. “Certain cardiac medications such as beta blockers can also cause dryness, and some diuretics like Lasix (furosemide) may dry out the eyes. We’re not telling patients to stop their cardiac or COPD medications, but we may contact their cardiologist or internist to let them know that the medication is causing dry eye and ask whether there are any other equally efficacious medications that the patient can try that won’t cause dry eye as a side effect.”
 |
Treating the OSD
In addition to withdrawing preservatives or other medications that may cause dry eye, clinicians turn to traditional OSD management approaches to alleviate glaucoma patients’ eye irritation. Experts say clinicians should consider not only the mechanisms and degree of OSD severity, but the individual patients’ abilities regarding treatment adherence.
Dr. Bournias says that “If patients are already taking glaucoma drops, it can be difficult for them to add yet more drops to treat their dry eye. Instilling the drops may be physically difficult, and adding more drops to treat OSD may decrease their compliance due to the complexity of the regimen and the irritation caused by the drops themselves if they contain preservatives.”
He says that if a patient hasn’t been treated for glaucoma yet and the glaucoma is mild or recent onset, he may treat the ocular surface before initiating glaucoma treatment. “We would, of course, initiate the treatment right away if the pressures were really high or the glaucoma were more advanced,” he notes.
Here are some options for treating glaucoma-related OSD:
• Artificial tears. Dr. Bournias says he likes to start simple. “I begin with lubricating tears and give patients samples of what’s available in the office,” he says. “I often step it up to preservative-free artificial tears that come in vials.”
Dr. Rapuano says, “We often recommend that our G-OSD patients instill an artificial tear drop five minutes before instilling glaucoma medications so that their eye is less susceptible to irritation. They can continue to use the artificial tears five minutes later to bathe the eye. Additionally, if the OSD is severe, it can affect a glaucoma patient’s perimetry. We usually overcome this by giving the patient some drops before and during the visual field test.”
• Anti-inflammatory medications. “When co-managing glaucoma patients, we’ll often switch them from tears with preservatives to tears without preservatives, or prescribe anti-inflammatory medications such as Restasis (cyclosporine 0.05%, Allergan), Cequa (cyclosporine 0.09%, Sun Pharmaceuticals) or Xiidra (lifitegrast 5%, Novartis),” notes Dr. Rapuano.
“These medications help patients produce more tears by controlling inflammation,” explains Dr. Bournias. “I tell patients that the prescription medications can take time to work, and that they may still need to use artificial tears—hopefully less frequently or not at all. Patients may have a better response to artificial tears once they’re on the prescription medication. Xiidra and Restasis [and Cequa] don’t have BAK, of course, so while this would add a drop to the regimen, it wouldn’t cause further inflammation.”
• Punctal occlusion. “Punctal plugs are easy, safe and tend to be covered by insurance,” says Dr. Bournias. “However, if the patient has a significant amount of OSD with MGD or blepharitis, I generally won’t use punctal plugs until the ocular surface is cleaned up, because using plugs early on will block the drainage of the tear film. That inflamed tear film has oil buildup, mucus and other inflammatory debris, and we don’t want that material staying on the eye. The ocular surface should be clean before occluding the punctum with a plug.”
• Steroids and antibiotics for lid inflammation. As long as he’s able to closely monitor the glaucoma patient, Dr. Bournias says he feels comfortable using a mild steroid for a short period of time (one to three weeks) such as Lotemax SM (loteprednol etabonate 0.38%, Bausch+Lomb), Eysuvis (loteprednol etabonate 0.25%, Kala Pharmaceuticals) or fluorometholone. “Don’t be afraid to get the inflammation under control at the outset,” he says. Antibiotic drops such as fluoroquinolone and ofloxacin are also useful for clearing up the lids if there’s bacterial buildup, Dr. Bournias adds. “Azasite (azithromycin 1%, Akorn) is quite nice because it’s thick,” he says. “I prefer the thicker drops because they tend to stay on the lid. Ointments such as azithromycin or tobramycin/dexamethasone, if you want a steroid effect, can be applied at night since they blur the vision.”
• Lid hygiene. “If steroids or antibiotics don’t work sufficiently or the patient needs further therapy, we tend to turn to hygiene treatment,” he continues. “We instruct the patient to clean their lids with a Q-tip, warm water and baby shampoo, or Ocusoft Lid Scrub or another type of lid scrub. Sometimes we recommend a spray such as Avenova (hypochlorous acid 0.01%) to clean the lid. We might even try low-dose doxycycline pills—20 to 50 mg, or even 100 mg, depending on the patient’s tolerance. We usually have patients on doxycycline for a few months or more. Doxycycline is useful because it pulls oil out of the lid as a side effect.”
He says his practice is very diligent about teaching patients the proper way to clean their lids. “We constantly go over this with our patients,” he says. “They come back, and if they’re not doing well enough, we quiz them and ask them to demonstrate their technique.”
Dr. Rapuano notes that “Patients’ blepharitis may not be a result of chronic glaucoma medication use, but if the eyes are extra sensitive or the glaucoma medications are particularly toxic, such as those containing higher concentrations of benzalkonium chloride, then any additional help to clear up the blepharitis may be beneficial.”
• Omega-3s. Dr. Salim points out that while the DREAM Study didn’t find a significant benefit to using omega-3 supplements vs. olive oil placebo, another prospective, multicenter study (sponsored by Spain’s Brudy Laboratories, maker of products containing omega-3s) did report a benefit of omega-3s in glaucoma patients with OSD.1 “Supplementation of 1,500 mg of omega-3 fatty acids per day over 12 weeks reduced DED symptoms and improved Schirmer’s and TBUT scores in patients on topical glaucoma drops,” she says.
• Amniotic membrane. Dr. Bournias says amniotic membrane is a well-tolerated, simple approach for addressing OSD that’s usually covered by insurance. “It’s placed on the patient’s eye and held in the fornix,” he explains. “We leave it in for a few days, and after it’s removed, the AM effect continues for a few weeks. It stimulates stem cells along the limbus and results in a nicer-looking cornea. Patients tend to tolerate their glaucoma drops better after AM, and their artificial tears tend to be more effective.”
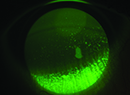
 |
| Significant punctate keratitis and tear film abnormality in a dry-eye patient with glaucoma. Findings such as these are common in patients who are using topical glaucoma medications that contain preservatives. |
Bypassing the Ocular Surface
Some newer medical modalities for glaucoma and dry eye avoid the cornea altogether. “One I’ve been using lately is Durysta (Allergan), a bimatoprost implant that’s injected into the anterior chamber,” Dr. Bournias says. “It can be done at the slit lamp, and it’s well-tolerated and covered by insurance. The only issue now is that it’s approved as a one-time application; hopefully it’ll be approved for use as an on-going treatment.”
The ARTEMIS 1 study comparing Durysta to timolol reported noninferiority over a 12-week period, with up to two additional administrations at four-month intervals. “This sustained-release implant may be particularly beneficial to glaucoma patients with OSD because it can give their ocular surfaces a rest from topical bimatoprost,” says Dr. Salim. “We can work toward minimizing their inflammation and improving their ocular surface during this period with preservative-free drops or even glaucoma surgery.”
Tyrvaya (varenicline solution 0.03 mg, Oyster Point Pharmaceuticals) is a recently approved, twice-daily nasal spray for DED. Marjan Farid, MD, a clinical professor of ophthalmology and director of the cornea, cataract and refractive surgery program and the ocular surface disease program at UC Irvine School of Medicine, says the cholinergic agonist works by stimulating the trigeminal nerve. “This provides a pathway for parasympathetic stimulation of the entire lacrimal-functional unit,” she says. “It helps the body to naturally produce all of the components of a healthy tear film and restore homeostasis.
“The Phase III studies, ONSET 1 and ONSET 2, both met their primary endpoints,” Dr. Farid notes. “At the end of four weeks, they reported significant improvement in the percentage of patients who had more than 10 mm of change in their Schirmer’s score, compared to the vehicle arm. They also saw a significant improvement in symptom scores in mild, moderate and severe DED patients. This improvement in symptom score was seen as early as week two in the treatment arm as compared to the vehicle. The main side effect was sneezing.
“This is an exciting new strategy for treating patients with DED,” she adds. “I’m looking forward to using this more in our patients and seeing how it fits into our dry-eye algorithm.”
Procedural Approaches
Procedures such as thermal pulsation and intense pulsed light are aimed at improving the patient’s own tear composition, specifically the lipid layer. “Thermal pulsation involves heating the lids to express oil and improve flow, and IPL selectively ablates vascular structures to reduce inflammatory mediators’ entry into the lid margin,” Dr. Farid explains. “You really want to give these patients a preservative-free way of naturally stimulating their own lacrimal-functional unit. Rather than using yet another drop on the ocular surface, it’s the patient’s own lipid layer and improved quality of tears that are helping to restore homeostasis. Natural tears have vitamins, growth factors and more than 1,500 proteins, unlike artificial tears. Artificial tears don’t restore these essential components.”
Dr. Farid uses LipiFlow (Johnson & Johnson Vision) for lipid layer restoration and the TearCare System (Sight Sciences) to express meibomian glands. “I see good results with both,” she says. “With TearCare, I’m able to titer the pressure to evacuate the oil glands and tailor the treatment to the patient’s degree of MGD.”
Laser Trabeculoplasty
“Laser treatments may be beneficial to G-OSD patients, especially if we’re unable to get them off preservatives, they’re on the maximum number of medications or their ocular surface is really terrible,” says Dr. Rapuano. “We’re always trying to reduce the drop load.”
“The LiGHT trial demonstrated that selective laser trabeculoplasty is a suitable first-line treatment for glaucoma and ocular hypertension,” says Dr. Salim. “After SLT, a higher percentage of patients achieved target IOP, fewer patients required glaucoma surgery and 74.2 percent of eyes required no drops at 36 months. SLT is also suitable for repeat treatments.”
Dr. Bournias says he offers laser trabeculoplasty early on. “We think that by treating patients early on with SLT, they’ll do better in the long term, because even if the laser trabeculoplasty doesn’t work effectively over the course of five or 10 years—say they get only a one- or two-point drop—that extra 1 to 2 mmHg might be of some value over the patient’s lifetime.”
If SLT isn’t effective, he offers ALT. “ALT isn’t done as much anymore, but it’s still an option,” he says. “Though it’s not as repeatable as SLT, it often works when SLT doesn’t. I’ve seen this happen in many patients. The effects of ALT also seem to last longer.”
MIGS
“There are several MIGS procedures available now that have great potential for reducing our glaucoma patients’ drop burden and the ocular toxicity resulting from chronic drop use,” says Dr. Salim. “The COMPARE study reported an average reduction of 1.6 drops after Hydrus implantation and one drop after iStent Inject implantation (p=0.004). We’ve also seen reports of reduced OSD symptoms after combined MIGS and cataract procedures, likely due to the need for fewer postoperative medications.
“Because of the associated reduction in drops, and thus ocular toxicity, performing MIGS earlier may help to minimize subconjunctival scarring and optimize surgical outcomes should patients need bleb-based surgery down the line,” she continues. “MIGS procedures may also avoid some of the side effects associated with traditional glaucoma surgeries. If patients don’t have visually significant cataract, SLT is a good alternative.
“Other surgical options that don’t require concomitant cataract removal include goniotomy, GATT, Trabectome and viscocanaloplasty,” she adds.
Dr. Salim is a consultant and speaker for Aerie Pharmaceuticals. Dr. Rapuano is a consultant for Bio-Tissue, Dompé, Glaukos, Kala, Oyster Point, Sun Ophthalmics, Tarsus and TearLab. Dr. Farid is a consultant for Allergan, Bausch + Lomb, BioTissue, CorneaGen, Dompé, Tarsus, Orasis, Johnson & Johnson Vision, KALA, Novartis, Sun Pharmaceutical and Zeiss. Dr. Bournias has no related financial disclosures.
1. Tellez-Vazquez J. Omega-3 fatty acid supplementation improves dry eye symptoms in patients with glaucoma: Results of a prospective multicenter study. Clin Ophthalmol 2016;10:617–626.