|
Predisposing conditions are important in determining a patient’s risk for endogenous endophthalmitis. In patients with acute or chronic panuveitis of unclear origin, invasive diagnostic procedures, most commonly pars plana vitrectomy, may be necessary to make the diagnosis. Identified risk factors for endogenous endophthalmitis include: chronic diseases (e.g., diabetes mellitus, renal failure, malignancies and acquired immunodeficiency syndrome); immunosupressive treatment; recent invasive surgery; intravenous drug abuse; indwelling catheter; endocarditis; hepatobiliary tract infections; organ transplantation; pregnancy or delivery; genitourinary surgeries; or dental procedures.4 Eliciting a history of intravenous drug abuse is especially important and often difficult given patients’ reluctance to discuss this issue. Positive history of underlying medical conditions such as diabetes, cardiac disease and malignancy was reported in 90 percent of patients in a report by Annabelle A. Okada and colleagues in 1994.5 A major review of endogenous endophthalmitis patients reported underlying medical conditions predisposing to ocular infection in 56 to 68 percent of cases.6 Zenith H. Wu and colleagues reported identification of preexisting predisposing condition in 90.9 percent of patients and the most common systemic condition found was diabetes mellitus (50 percent).7
|
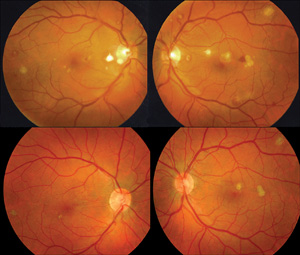
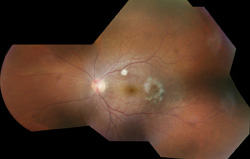
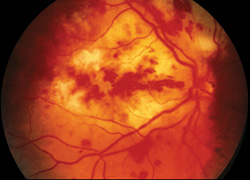
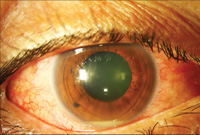
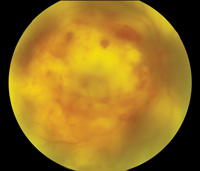
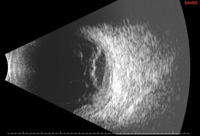
The involved eye may have pain, redness, floaters or decreased vision. Diagnosis is delayed in the pediatric population by inability to report symptoms early. Bilateral presentation is reported in 14 to 25 percent of cases and more commonly with fungi and bacteria like Meningococcus, Escherichia coli and Klebsiella.6 Endogenous endophthalmitis may be unilateral to begin with and subsequently develop in the fellow eye. Bacterial or fungal infections may exhibit iris microabscess, hypopyon, varying grades of vitreous haze, discrete retinal nodules, perivascular retinal hemorrhage, arteriolar emboli or necrotic retina. Severe cases may progress to panophthalmitis. Presence of chocolate brown exudate in the anterior chamber suggests Bacillus as the responsible organism, while Listeria is characterized by brown hypopyon and Serratia by a red hypopyon.
Fungal endogenous endophthalmitis with Candida may have fluffy balls in the vitreous, chorioretinitis, hypopyon, perivasculitis, optic neuritis or chorioretinal lesions (creamy, deep and well-circumscribed). Infection with molds like Aspergillus is more fulminant, typically confined to the subretinal space and may have chorioretinal lesions (confluent with indistinct margins), intraretinal hemorrhages, vascular occlusion or full thickness retinal necrosis. Positive vitreous aspirate cultures are more difficult to obtain from molds than yeasts, perhaps because molds do not involve the vitreous as commonly as yeasts.13
Endogenous endophthalmitis may mimic conjunctivitis, non-infectious anterior uveitis, iritis, acute glaucoma, cellulitis, cataract and, especially, retinoblastoma in children. Misdiagnosis at initial presentation has been reported in 16 to 63 percent of cases, thus delaying the diagnosis and proper management.6,12
The diagnosis of endogenous endophthalmitis is typically made following microbiologic evidence of infection from an intraocular sample (aqueous or vitreous). Positive cultures from blood, cerebrospinal fluid or any extraocular site can be highly suggestive. Blood culture positivity rate varies widely, from 33 percent to 94 percent of cases.6,8 In the absence of localizing symptoms the diagnostic yield from blood in endogenous Aspergillus endophthalmitis is reported to be very low.14 Cultures should be set up with both aerobic and anaerobic medium (chocolate agar, sheep blood agar and Sabouraud agar) and incubated for up to two weeks. Gram stain is commonly used to assess for bacteria. Fungal growth can be confirmed by Geimsa or Calcorfluor white stains. Polymerase chain reaction study of the tissue sample is a quick method to identify responsible organisms but has a drawback that it could not be used to assess antibiotic or antifungal sensitivity.
|
Treatment
• Bacterial endogenous endophthalmitis. Soon-Phaik Chee and colleagues reported that systemic antibiotics can achieve therapeutic levels in the eye due to the disrupted blood ocular barrier.1 Nevertheless, systemic agents are most often supplemented with intravitreal antimicrobials and vitrectomy, especially in the setting of prominent vitreous involvement. Vancomycin (1 mg/0.1 mL) and ceftazidime (2.25 mg/0.1 mL) remain the intravitreal antibiotics of choice. According to a review study, eyes undergoing pars plana vitrectomy are three times more likely to retain useful vision than those who did not undergo vitrectomy.6 Also these eyes are three times less likely to require evisceration or enucleation. Broad-spectrum systemic antibiotics like vancomycin, ciprofloxacin, aminoglycosides or third-generation cephalosporins made up the mainstay of treatment previously but are now used adjunctively to local therapy. Recommended antibiotics are wide-spectrum antibiotics which cover most of gram positive and negative organisms. Systemic antibiotics are typically continued for at least three to four weeks or as the extraocular infection of the patient dictates.
• Fungal endogenous endophthalmitis. Treatment depends on the extent of ocular involvement. Systemic therapy alone is sufficient when the infection is isolated to the retina and choroid. Vitrectomy and intravitreal antifungal injections along with systemic therapy are recommended in cases where vitreous is involved.15
Intravenous amphotericin-B has classically been the drug of choice but needs careful monitoring in view of its systemic toxicity. Oral voriconozole or fluconazole in conjunction with local therapy are increasingly used. Intravitreal injection of either voriconazole (100 μg/0.1 ml) or amphotericin-B (5 to 10 μg/0.1 ml) ensures immediate, adequate levels of antifungal agent in the posterior segment. Voriconazole may have better coverage for Aspergillus species and some Candida species (like C.glabrata and C.krusei) where fluconazole or amphotericin-B are ineffective. Newer antifungal agents like posaconazole, echinocandins, micafungin, caspofungin and anidulafungin have poor ocular penetration and are not recommended for use in endophthalmitis.16 Vitrectomy helps in decreasing the load of infection and better accessibility of antifungal agents to intraocular structures. The need for repeat intravitreal injections should be based on clinical improvement, status of the eye (vitrectomized vs non-vitrectomized) and pharmacokinetics of the antifungal medicine.
Local or systemic corticosteroids are generally avoided in fungal endophthalmitis,10 although their use remains controversial.14,15
Outcome
Endogenous endophthalmitis cases usually have a poor visual outcome. Visual acuity of counting fingers or more is reported in 22.2 to 41 percent cases.17,18 Loss of vision due to blindness, evisceration or enucleation is reported in 55 to 69 percent of cases.6 Visual outcomes after treatment are worse for endogenous Aspergillus endophthalmitis as compared to Candida cases, and it could be due to earlier detection of Candida infection, leading to more timely initiation of treatment.15
Patients who have extraocular foci of bacterial infection have a reported mortality rate of 5 percent6 to 32 percent.19 Factors such as infection with virulent organisms, poor host defense, misdiagnosis leading to delayed treatment, inadequate treatment, use of inappropriate antibiotics, panophthalmitis are considered to be associated with poor prognosis. Fungal infection has high mortality, with a 7-percent reported rate of mortality in systemic Candida infection.20
Endogenous endophthalmitis is a potentially devastating eye infection and needs to be diagnosed and managed promptly. Use of combined ocular and systemic antibiotics is common. Systemic co-infection is common and is associated with a high rate of mortality.
Dr. Relhan is a research fellow in vireoretinal diseases and uveitis at Bascom Palmer Eye Institute. She was previously a junior consultant in vitreoretinal surgery at LV Prasad Eye Institute in Hyderabad, India. Dr. Albini is an associate professor of clinical ophthalmology specializing in vitreoretinal diseases and uveitis at Bascom Palmer Eye Institute. Dr. Flynn is a professor of ophthalmology specializing in vitreoretinal surgery at Bascom Palmer. Inquiries should be directed to Dr. Albini at TAlbini@med.miami.edu.
1. Chee SP, Jap A. Endogenous endophthalmitis. Curr Opin Ophthalmol 2001;12(6):464-70.
2. Rachitskaya AV, Flynn HW, Davis JL. Endogenous endophthalmitis caused by salmonella serotype B in an immunocompetent 12-year-old child. Arch Ophthalmol 2012;130(6):802-4. doi: 10.1001/archophthalmol.2011.1862.
3. Chaudhry IA, Shamsi FA, Al-Dhibi H, Khan AO. Pediatric endogenous bacterial endophthalmitis: Case report and review of the literature. J AAPOS 2006;10(5):491-3. Epub 2006 Sep 7.
4. Flynn HW Jr. The clinical challenge of endogenous endophthalmitis. Retina 2001;21(6):572-4.
5. Okada AA, Johnson RP, Liles WC, D’Amico DJ, Baker AS. Endogenous bacterial endophthalmitis - Report of a ten-year retrospective study. Ophthalmology 1994;101:832-8.
6. Jackson TL, Eykyn SJ, Graham EM, Stanford MR (2003). Endogenous bacterial endophthalmitis: A 17-year prospective series and review of 267 reported cases. Surv Ophthalmol 2003;48(4):403-23.
7. Wu ZH, Chan RP, Luk FO, Liu DT, Chan CK, Lam DS, Lai TY. Review of Clinical Features, Microbiological Spectrum, and Treatment Outcomes of Endogenous Endophthalmitis over an 8-Year Period. J Ophthalmol 2012:265078. doi: 10.1155/2012/265078. Epub 2012 Feb 23.
8. Schiedler V, Scott IU, Flynn HW Jr, Davis JL, Benz MS, Miller D. Culture-proven endogenous endophthalmitis: Clinical features and visual acuity outcomes. Am J Ophthalmol 2004;137:725-31.
9. Wong JS, Chan TK, Lee HM, Chee SP. Endogenous bacterial endophthalmitis: an east Asian experience and a reappraisal of a severe ocular affliction. Ophthalmology 2000;107:1483-91.
10. Lingappan A, Wykoff CC, Albini TA, Miller D, Pathengay A, Davis JL, Flynn HW Jr. Endogenous fungal endophthalmitis: Causative organisms, management strategies, and visual acuity outcomes. Am J Ophthalmol 2012;153:162-6.e1. doi: 10.1016/j.ajo.2011.06.020. Epub 2011 Sep 13.
11. Sridhar J, Flynn HW Jr, Kuriyan AE, Miller D, Albini T. Endogenous fungal endophthalmitis: Risk factors, clinical features, and treatment outcomes in mold and yeast infections. J Ophthalmic Inflamm Infect 2013;Sep 20;3(1):60. doi: 10.1186/1869-5760-3-60.
12. Binder MI, Chua J, Kaiser PK, Procop GW, Isada CM. Endogenous endophthalmitis: An 18-year review of culture-positive cases at a tertiary care center. Medicine (Baltimore) 2003;82(2):97-105.
13. Rao NA, Hidayat AA. Endogenous mycotic endophthalmitis: Variations in clinical and histopathologic changes in candidiasis compared with aspergillosis. Am J Ophthalmol 2001;132(2):244-51.
14. Weishaar PD1, Flynn HW Jr, Murray TG, Davis JL, Barr CC, Gross JG, Mein CE, McLean WC Jr, Killian JH. Endogenous Aspergillus endophthalmitis. Clinical features and treatment outcomes. Ophthalmology 1998;105:57-65.
15. Brod RD, Flynn HW, Miller D. Endogenous fungal endophthalmitis. In: Duane’s clinical ophthalmology. Hagerstown: Harper and Row; 2004. CD— ROM. ch 11
16. Bouza E1, Cobo-Soriano R, Rodríguez-Créixems M, Muñoz P, Suárez-Leoz M, Cortés C. A prospective search for ocular lesions in hospitalized patients with significant bacteremia. Clin Infect Dis 2000;30(2):306-12.
17. Riddell J 4th1, Comer GM, Kauffman CA. Treatment of endogenous fungal endophthalmitis: Focus on new antifungal agents. Clin Infect Dis 2011;52(5):648-53. doi: 10.1093/cid/ciq204. Epub 2011 Jan 16.
18. Greenwald MJ, Wohl LG, Sell CH. Metastatic bacterial endophthalmitis: A contemporary reappraisal. Surv Ophthalmol 1986;31(2):81-101.
19. Chen YJ1, Kuo HK, Wu PC, Kuo ML, Tsai HH, Liu CC, Chen CH. A 10-year comparison of endogenous endophthalmitis outcomes: An east Asian experience with Klebsiella pneumoniae infection. Retina 2004;24(3):383-90.
20. Menezes AV1, Sigesmund DA, Demajo WA, Devenyi RG. Mortality of hospitalized patients with Candida endophthalmitis. Arch Intern Med 1994;154(18):2093-7.