With that in mind, I’d like to share some of my post-surgery experience with thousands of these patients over the years, in hopes that some of what I’ve learned may help other surgeons and patients achieve the best possible outcomes.
Follow-up (When All Is Well)
Once the trabeculectomy itself is complete, I usually see the patient the following day and then a week later to adjust the releasable sutures. After that, depending on the IOP, I’ll see the patient either one or two weeks later, because by three weeks—except in a hypotonous situation—all the conjunctival and releasable sutures are usually out.
I aim to keep the IOP at around 15 to 20 mmHg on postop day one; this allows vision to return to normal (or close to normal), so the patient can resume daily activities. In most cases pressure is slightly elevated relative to the goal, which is ideal at that point because we want to avoid hypotony. Depending on my pressure goal, a releasable suture can be cut as early as the first day or within the first three weeks. (My goal is usually a 50-percent drop in IOP.)
Suture removal is done at the slit lamp with a needle or a blade, and jeweler’s forceps. Occasionally, if the pressure is elevated, I’ll use a laser with either a Blumenthal or Hoskins lens to cut the internal sutures. However, I usually reserve the laser for later in the postoperative period, because in most cases the pressure can be controlled by removing a single releasable suture; the flow is increased immediately and the IOP comes down.
I generally keep the patient on antibiotic drops for a week, with steroids q.i.d. for two weeks, t.i.d. for two weeks, b.i.d. for two weeks, and once a day for two weeks before stopping. I also prescribe an NSAID once a day for at least six weeks. (Note: I don’t prescribe an NSAID with the steroid if the eye has a lot of corneal epithelial irregularity.)
| ||||||||||
One reason I continue NSAIDs for this length of time is that I believe that low levels of inflammation, leading to scarring, are a significant cause of trabeculectomy failure. This is something that might not be easily noted at the slit lamp, but the NSAID will help to prevent or alleviate it. We also encourage the patient to use tears, to keep the eye comfortable.
If all is well at three weeks, I’ll see the patient again in a month, or he’ll return to the referring doctor. If all is not well, then I see the patient as often as is needed.
Postop Examination
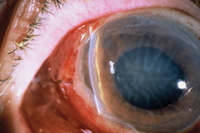
When you see the patient on the first day postop, it’s crucial to be a careful observer, because there’s no other way to be sure everything is as it should be. For example, if the patient’s vision is perfect on postoperative day one, that usually means the pressure is not low enough. On the other hand, blurry vision could mean a number of different things. Perhaps the pressure is too low, or at the expected level, or perhaps even too high with corneal edema or with blood blocking the osteum, causing the pressure to rise. That’s why it’s critical to carefully check the cornea, the anterior chamber depth, the amount of inflammation and subconjunctival hemorrhage and how much blood is near the superior flap.
|
Signs you want to look for on the first day postop include corneal edema, indicating that the pressure’s too high, and endothelial folds, which indicate that the pressure’s too low. The latter is usually associated with shallowing of the anterior chamber.
Managing a Shallow Chamber
Shallowing of the anterior chamber often occurs when the IOP goes from high to low. Simultaneously, you may see the development of a choroidal detachment, fluid in the suprachoroidal space that pushes the lens-iris diaphragm forward and further flattens the chamber. It’s a vicious cycle, because one feeds into the other.
A shallow anterior chamber calls for different levels of response, depending on the severity. The severity is graded at four levels: Grade 0 means the chamber is shallow but the endothelium isn’t touching any other tissues, even at the periphery; grade 1 suggests that there’s peripheral touching, but the chamber is still formed everywhere else. If the chamber is between grades 0 and 1, we just follow the patient carefully, seeing him at one week and every week thereafter.
Grade 2 implies that the endothelium is touching the mid-iris but not the pupil; grade 3 means the endothelium, lens and iris are all touching. Grade 2 is very serious because it can get worse and become grade 3. To restore the anatomy and remove the threat of a grade 3 chamber, we usually reform the chamber at the slit lamp, with informed consent from the patient.
|
Whenever there’s a shallow chamber, you should dilate the pupil to move the lens-iris diaphragm back. Moving the lens-iris diaphragm backwards may jump-start the ciliary body to make more aqueous, thereby deepening the chamber and raising the pressure. About 80 percent of the time this will cause the choroidal detachment to disappear. To accomplish this, we usually use atropine 1%, two to three times a day. (However, we avoid atropine in patients who have prostate issues.)
We always check to see if there are choroidals when the pressure is low; if there are, we explain to the patient that 80 percent of the time this will heal by itself. We monitor the patient every week or every two weeks, if the chamber is not excessively shallow.
About 20 percent of the time we need to intervene and drain the choroidal detachment in the OR.
Doing so usually encourages the ciliary body to produce more aqueous, reestablishing the anterior chamber depth and improving vision. In addition, we usually increase the steroids and make sure there’s no leak causing the hypotony. Then, we always instruct the patient not to rub the eye and to use a shield at night. All these preventive measures should encourage the eye to become pressurized once again. Because this is often a long, drawn-out affair, we want to prevent this whole cycle by keeping the pressure between 15 and 20 mmHg early on in the postoperative period.
|
On the other hand, sometimes a shallow chamber catches us by surprise; there doesn’t seem to be a lot of flow when we check the bleb, but in the postoperative period the pressure is lower than we expected. In fact, sometimes the eye shuts down altogether when the pressure starts very high and drops after surgery. The eye may simply stop making aqueous, causing the pressure to drop very low despite all of our efforts.
Fortunately, most of the time we do have a good idea which patients are at risk of hypotony, and we make every effort to avoid that outcome during the surgery. In general, I’d say we encounter hypotony about 5 percent of the time.
Managing Hemorrhages
Hemorrhages are a part of life when performing trabeculectomy. That’s partly because most of these patients are older individuals who are on blood thinners such as Plavix, Coumadin or just aspirin—sometimes a combination—for various systemic reasons, most of which are pretty serious. In my practice, I don’t have patients stop taking their blood thinners. But I also don’t use peribulbar blocks, so there’s a bit of a safety factor there, in that we’re not giving any needles in locations where we can’t see what’s happening. Instead, we use a blitz anesthesia technique combining topical lidocaine 2% jelly, intracameral nonpreserved 1% lidocaine and subconjunctival lidocaine. That strategy allows us to stop any bleeding because we can see it if it happens.
Sometimes when the IOP goes from high to low a patient will develop a suprachoroidal hemorrhage instead of just a choroidal detachment, which is very serious. In fact, a suprachoroidal hemorrhage can often be diagnosed over the phone. The patient will be sitting in the kitchen glancing up at the clock, and exactly at 10:02, she’ll experience extreme pain in the eye. The patient can often tell you exactly what she was doing when it occurred.
|
When this occurs, I usually have a retina colleague check out the patient as well, to make sure there’s no vitreoretinal adhesions if the choroidals are kissing, which might lead to further retinal issues. Often, these hemorrhages need to be drained, but we usually wait 10 days for the blood to liquefy. If the choroidals are not kissing, then the glaucoma doctor usually drains them and reforms the chamber. If there’s any question of vitreoretinal adhesions, our retinal colleagues take over.
Managing a Bleb Leak
Ultimately, you want a low, diffuse, minimally vascular bleb without any leaks. In reality, of course, bleb leaks can occur early or late in the postoperative period, and there are a number of different ways to deal with them. In recent years there’s been a move toward fornix-based surgery, since it provides a more diffuse bleb; as a result, conjunctival sutures at the limbus are now part of the picture. These sutures occasionally leak, which can lead to a shallow anterior chamber, a hypotonous situation and failure of the bleb.
Any bleb leak requires intervention; the rate of endophthalmitis is 1 per-cent per year in the presence of a leaking bleb. For this reason, one of the steps glaucoma surgeons need to go through every time they examine patients post-trabeculectomy is checking for bleb leaks, usually using the Seidel test.
|
If a bleb leak occurs later in the postoperative period, it’s a very different matter. The seriousness of that type of leak depends on where the leak is, and how easy it is to fix.
Sometimes autologous blood injected into the dome of the bleb will help to close the hole. About 50 to 60 percent of the time a very large bandage contact lens such as the Kontur lens (Hercules, Calif.), can cover the hole. Occasionally, we initiate aqueous suppressants to decrease the flow and encourage the hole to heal. If the patient has to be brought back to the OR, conjunctival advancement will often help; compression sutures might also encourage it to heal.
During the healing process we have patients use antibiotic drops. However, I don’t recommend using them for a long time because over time they’ll self-select for bacteria that are resistant to the antibiotic.
Managing Infection
That brings us to a complication all surgeons dread: infection. Infection is always a serious matter, whether the bleb alone is infected (blebitis) or the chamber has become involved (endophthalmitis).
|
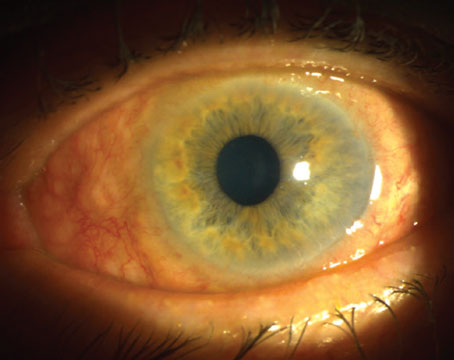
A hypopyon tells us that there may be endophthalmitis, probably originating from the bleb. When there are cells in the vitreous as well as the anterior chamber, the diagnosis is very clear. This is an emergency, because endophthalmitis can lead to a rapid downward spiral toward blindness.
When we observe these signs, an alarm goes out. In this situation the patient is treated with fortified antibiotics or fluoroquinolone every hour. If possible, the aqueous is cultured. A retina specialist usually sees the patient, and may perform a tap-and-inject treatment. The patient will have an ultrasound to determine the level of cells in the vitreous cavity.
Once it’s clear that the infection is under control, we begin steroids to quiet the eye down and hopefully save the bleb.
Post-surgery Patient Advice
Our advice to patients following trabeculectomy is routine: no eye rubbing, and they need to wear the shield for at least one week post-surgery. If there’s blood in the eye, I discourage any bending; the patient should sleep with extra pillows so the blood can settle inferiorly and then dissipate. We don’t encourage swimming during the first week or so while the stitches are still in place, or any weight-lifting or yoga during this period, although aerobic exercise is OK. Patients can resume showering, shaving and shampooing almost immediately after surgery.
Perhaps most important, we tell patients that if their vision changes, they should call us immediately. Since we’re worried about spontaneous bleeding, we’re worried about infection. Luckily, it’s rare that we get a call like this; more commonly we get a call reporting discomfort, tearing or scratching. But among those calls there are occasionally some very serious ones, so we always encourage our patients to call us immediately if they notice any significant change.
When a Bleb Is Failing
Unfortunately, bleb failure is not uncommon, no matter how perfectly the surgery is done. Usually we allow two to three months before deciding whether a bleb is a success or has failed. Obviously, if the pressure goes up at three to four weeks, we have to be on the lookout for continued rise in pressure, but this is often just a Tenon’s cyst that needs to soften, in which case we place the patient back on glaucoma medication and wait.
A bleb that’s failing can often be revived by needling it with mitomycin C. However, the best success with needling is usually long after the trabeculectomy has been established—usually a year or more. At that point, we have a 64-percent success rate of bringing a trabeculectomy back to life. If a trabeculectomy fails early, needling the bleb generally will not yield a successful result. In the meantime, if I notice that the eye is excessively red and there are blood vessels in the conjunctiva, and I’m fearful that the bleb will fail, I will sometimes give an Avastin injection into the bleb. (Of course, this is off-label.)
Occasionally the trabeculectomy will start to fail as early as six weeks. If that happens, depending on the health of the optic nerve and the visual field, we may need to move on to another management approach. In that situation, we usually wait until the eye totally quiets down, within about two months. Then we reassess the situation. If the trabeculectomy has definitely failed, I would consider an ExPress shunt on either side of the eye, if possible, or move on to a tube shunt if necessary.
Helpful Points to Remember
A few things to keep in mind when managing trabeculectomy patients:
• Make patients aware of potential complications before-hand. Patients who are going into the surgery with very high IOPs, for example, should be warned that they are at a greater risk of having the eye shut down and stop making aqueous postop. (You should also make it clear that you’re going to do everything possible to prevent this from happening.)
• Make sure the patient has realistic expectations regarding cataract. The patient needs to understand that a minimal cataract may progress following the trabeculectomy.
• Consider removing an existing cataract during the trabeculectomy. We often do this as a way to improve vision in addition to controlling the IOP, since cataract surgery in this select subpopulation is inevitable and is often best treated with a single trip to the OR.
• If the patient’s vision is perfect on postop day one, don’t assume all is well. As noted earlier, this probably indicates that the IOP is higher than it should be and needs to be addressed.
• Keep the focus on visual rehabilitation. It’s very important to get people back to their normal activities as quickly as possible, and not just focus on the IOP. Sometimes the patient’s refraction changes after the surgery, so you should discuss this with the patient beforehand.
Postop, we remove the releasable and conjunctival sutures by three weeks and encourage a re-refraction, if needed, as long as everything is stable. I like to make sure that patients are refracted and back to daily activities quickly, and engaging in all work activities by six to eight weeks at the latest.
• Be on the lookout for subtle bleb leaks. Not every leak is obvious, but the consequences can still be serious. So, always check for a leak, even if all seems well.
• Consider preplacing the scleral sutures before the sclerostomy. Trabeculectomies can sometimes trigger the development of against-the-rule astigmatism. One way we prevent that in the OR is by pre-placing the scleral sutures before taking out the block. That way, when the eye softens the sutures are in place, so we don’t induce any additional astigmatism.
• Consider not using an anesthetic block. This will avoid the possibility of unseen hemorrhages that might be caused by the injection.
Better Options: Coming Soon?
I’m tremendously encouraged by all of the research into ways to lower IOP by maximizing outflow without making a bleb. The problem is that so far there’s no one procedure that will lower IOP sufficiently in patients who need a very low, single-digit pressure—at least none reliable enough to become the new gold standard. So for now, trabeculectomy is still the go-to surgery for these patients. It’s imperfect, but when compared to blindness, the risks are definitely worth taking. REVIEW
Dr. Moster is a professor of ophthalmology at Thomas Jefferson University School of Medicine and an attending surgeon at Wills Eye Institute’s Department of Glaucoma.