Perhaps because the number of patients needing an iris prosthesis is so small, the technology in this arena has progressed slowly. Recently, however, several new options for replacing or supplementing a missing or damaged iris have appeared. One in particular that's not widely known in the
Better Appearance, Smaller Incision
Until recently, most iris prostheses only addressed the optical problems caused by iris damage: photophobia; aberration disorders; glare effects; and loss in depth of focus. They were not designed to mimic the patient's original iris. In fact, many iris prostheses are still only available in black, and most are rigid, requiring a large incision for implantation.
However, new options that address the need for a better cosmetic result—and a smaller incision—have recently begun to appear. One such device is a foldable prosthesis individually manufactured to match the appearance of a patient's original iris tissue: the CustomFlex (also known as the ArtificialIris) from HumanOptics. The CustomFlex is made of a pigmented, biocompatible, foldable silicone elastomer that's been used for many years in intraocular lenses. It's available in two versions—one containing a polymer fiber meshwork that allows the device to be sutured without tearing, and one without the meshwork. Both versions can be folded and implanted through a small incision. (The company recommends a 3.2-mm incision for the fiber-meshwork version, and a 2.5-mm incision for the fiber-free version.) The CustomFlex does not incorporate a refractive lens.
The prescribing surgeon provides the manufacturer with a high-quality photograph of the desired iris pattern; the color patterns in the final product are handmade and fully customized to match the photograph supplied, and the surface structure is designed to closely match the appearance of the natural iris. The surgeon also receives two standby implants with a slightly different color composition. The final match can be chosen based on viewing the prosthesis while still immersed in its sterile saline solution, compared to the remaining iris tissue. (One of the two standbys can also be used if the first choice becomes contaminated or damaged.)
The device comes as a complete 12.8-mm diameter iris with a 3.35-mm central aperture, a central circumference thickness of 0.4 mm and a peripheral thickness of 0.25 mm. It can be trimmed with a trephine or sharp scissors to custom fit the device as a segment sutured to an iris remnant, for transscleral support, or to reduce the diameter for a small eye.
The company recommends that if the eye is phakic, the crystalline lens should be replaced with an IOL, even if no cataract is apparent. The recommended placement of the CustomFlex is the ciliary sulcus, with a peripheral iridectomy. Contraindications to use of the CustomFlex include severe chronic uveitis, endothelial corneal dystrophy, microphthalmus, retinal detachment, untreated chronic glaucoma, rubella cataract, rubeosis of the iris and proliferative diabetic retinopathy.
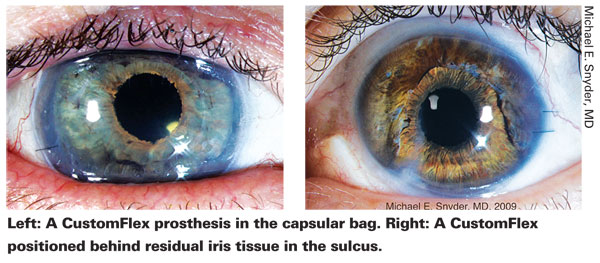
Working With the CustomFlex
Michael E. Snyder, MD, who practices at the Cincinnati Eye Institute and is a volunteer assistant professor at the University of Cincinnati School of Medicine, has implanted 30 of the CustomFlex devices so far and has also worked with most of the other available alternatives. Dr. Snyder says the CustomFlex has three significant advantages. "First, its realistic appearance is a big plus," he says. "The cosmetic results have been outstanding. One patient told me that two weeks after implantation his mother couldn't tell which iris had been damaged.
"Second, this device only requires a 2.75-mm incision," he continues. "That's important because most of the eyes we've implanted so far have congenital aniridia syndrome; so the epithelial cells on the surface of the cornea—where we make our incision—are particularly vulnerable. Furthermore, for individuals who have suffered trauma, a large incision increases the likelihood of intraoperative complications and could compromise future glaucoma surgery.
"A third advantage of this product is that it's made of a flexible material, so a stitch can be passed through it," he adds. "In some patients, fixation of the iris element to the wall of the eye may be necessary, either at the time of implantation or later. That's something you can't do with any other device." Dr. Snyder adds that so far he hasn't seen any downside to the prosthesis.
To order a CustomFlex Dr. Snyder takes a picture of the remaining uninjured iris, if possible. "If both irises are gone, we ask the patient to take a picture of someone else's iris that would be a desired appearance match for them," he says. "One 7-year-old aniridic child that we worked on chose his mother's iris."
Dr. Snyder says that the Europeans have been implanting this device primarily into the ciliary sulcus. "When there's an intact capsular bag, I place the device in the bag while performing the cataract surgery," he says. "If there's no intact capsular bag, I place the device in the sulcus and usually use suture fixation to guarantee a lack of movement.
"When the patient has a partial iris defect," he continues, "many European surgeons cut a small segment of the CustomFlex and stitch it to the remaining iris with very nice results. In my experience, the cosmetic appearance is better when I place the entire diaphragm behind the remaining iris. Either way is acceptable."
A Variety of Options
Dr. Snyder has also worked with most of the other iris prostheses that are available.
"Most ophthalmologists are aware of the Morcher products, owing to their longer history in the
"Ophtec offers several options as well," he continues. "One is a 9-mm-diameter rigid device with a central opening of 4 mm; it can be ordered with an optic filling the central space and requires a 9-mm incision. That model has a smooth surface and comes in three colors: light blue; light brown and light green. Ophtec also has a device that comes in five different pieces that can go through an approximately 5-mm opening. I've implanted four or five of them, but I've found that getting all of those pieces oriented inside the eye is fairly challenging. The incision size is a little smaller than the incision needed for the rigid diaphragm devices."
In terms of a timetable for receiving FDA approval of the CustomFlex prosthesis, Dr. Snyder says that HumanOptics plans to begin clinical trials soon. For more information, visit artificial-iris.com.
Dr. Snyder has no shareholder stake in the CustomFlex, but does consult for HumanOptics and will be principal investigator if the CustomFlex goes into clinical trials for U.S. Food and Drug Administration approval. He is an investigator in the ongoing Ophtec 311 iris prosthesis study.




