In Western countries, infectious conjunctivitis is common, with an incidence of 15 per 1,000 patients per year in primary care.1 Although most incidents of conjunctivitis are treated in the primary care setting, complications from viral or bacterial infection can arise and lead to sight-threatening conditions.2 Conjunctivitis is also a major health issue in developing countries, especially in areas with poor public water supplies. Outside the United States, cases of conjunctivitis are estimated to affect more than 30 million people, due to both the highly contagious nature of infectious forms and to poor sanitary conditions that lead to cycles of repeated infection. An example of this is trachoma, a bacterial conjunctivitis that is the most preventable cause of blindness worldwide; it’s estimated to be responsible for ~2 million cases of blindness or visual impairment.3 While rare in the United States, the devastating effects of trachoma and other conjunctival infections serve to remind us that these seemingly innocuous ailments can have serious complications. This month we examine the current standard of care and emerging trends in this all-too-common ocular malady, with a particular focus on viral forms of the disease.
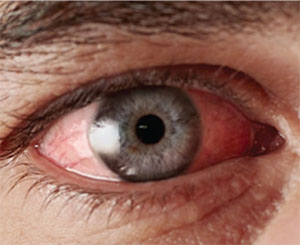 |
| Steroid treatment in the setting of viral conjunctivitis may increase the latency of the virus, prolonging the infection. |
Identifying Infectious Agents
Infectious conjunctivitis is particularly prevalent in the pediatric population, where one in eight schoolchildren is expected to have an episode each year.4 The contagious nature of these infections means that children diagnosed with “pinkeye” are required to leave school and seek treatment. Indeed, cases of conjunctivitis have been shown to be the leading cause of day care and school absences, which directly impacts a parent’s time at work.5 Outbreaks tend to be rapid and pervasive, owing to the extremely contagious nature of the condition; the importance of rigorous hygiene cannot be overemphasized in these situations. This aspect of the disease leads to a major impact on productivity through losses of time at work and medical visits that represent a significant economic burden for society.
Patients presenting with infectious conjunctivitis typically experience a bilateral hyperemia along with other signs such as ocular discharge, tearing, chemosis, itching, pain or irritation. Some patients may also experience lid edema or photophobia, petechial hemorrhage, follicles on the tarsal or fornix conjunctiva, and pre-auricular lymphadenopathy.6-9 Other signs associated with a viral rather than bacterial infection are palpable pre-auricular or submandibular lymph nodes.7 Viral infections may also produce follicles caused by a lymphocytic response of the conjunctiva.7 It’s important to note that these follicles are different from papillae, which are formed by bunches of conjunctival capillaries that become dilated during some other ocular diseases such as giant papillary conjunctivitis.
While there are many signs and symptoms that overlap between bacterial and viral forms of conjunctivitis, the nature of the discharge is typically the key diagnostic characteristic: To differentiate between viral and bacterial forms of conjunctivitis, it’s generally accepted that a thick, purulent discharge is associated with bacterial conjunctivitis, while a watery discharge is more characteristic of viral conjunctivitis.7,8 Specifically, if the eye exhibits a sticky, opaque secretion, then a bacterial infection is most likely; without this, a viral infection is most probable. Laboratory tests confirming these diagnoses are not usually required, but a culture swab of the conjunctiva prior to initiation of treatment may be useful.10 The use of an in-office rapid antigen test can help prevent the inappropriate use of antibiotics, due to its accuracy in identifying a viral infection from a group of viruses—adenoviruses—which comprise 65 to 90 percent of all viral conjunctivitis cases.10 The in-office antigen test has been shown to be highly specific (~95 percent), although the reports of test sensitivity have been more variable.11,12 The value of these tests is that they prevent the unnecessary use of antibiotics, which have no value in our therapies for viral infections.
Although many patients visit a physician with the hopes of receiving a treatment for quick recovery, there is currently no specific treatment for the viral form of infectious conjunctivitis. Treatment typically involves the use of cold packs, artificial tear lubricant eye drops, ocular decongestants and education on preventing transmission of the virus through frequent hand-washing.10,13 For the most part, antivirals have been shown to be ineffective in treating viral conjunctivitis; the exception to this is in the case of herpes simplex viral infections, which comprise between 1 and 5 percent of all acute conjunctivitis cases. Antivirals such as acyclovir, trifluridine and valacyclovir have all been shown to effectively treat herpesvirus infections.9
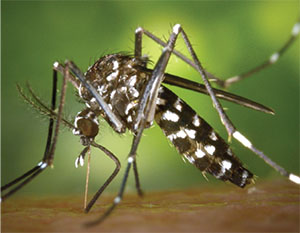 |
| Aedes aegypti, the mosquito that carries the Zika virus. Among its many other signs and symptoms, Zika can also cause conjunctivitis in infected individuals. |
Beyond Adenovirus
Although the majority of viral infections are due to members of the adenovirus family, there is a tremendous diversity in the types of viruses that infect the eye.13 There are more than 40 types of adenovirus that cause conditions including colds, gastrointestinal illness, conjunctivitis or more serious ocular conditions. Ocular manifestations of adenoviral infections include pharyngoconjunctival fever, epidemic keratoconjunctivitis, acute nonspecific follicular conjunctivitis, and chronic kerato-conjunctivitis. Other viruses known to cause conjunctivitis include the human immunodeficiency virus, Varicella zoster virus and Epstein-Barr virus.12,13
Another virus that has been the subject of much public debate in the past year is the Zika virus.14 Zika is a mosquito-borne RNA virus, related to viruses associated with dengue fever, yellow fever and West Nile virus. The virus gained public attention during the Brazil Olympics because of its association with congenital abnormalities in infants of infected mothers. Most patients infected with Zika are asymptomatic, but those infected individuals who display disease features experience a constellation of symptoms including conjunctivitis, fever, maculopapular rash and joint pain.14 Although the disease is still rare in the United States, increased Zika incidence rates in some southern states suggest that Zika infection may need to be considered as a possible cause for cases of conjunctivitis of unknown etiology that occur in those regions.
Beyond its association with conjunctivitis, a mouse model of Zika demonstrated that the virus was present in the tears, suggesting that Zika might be secreted from the lacrimal gland or shed from the cornea.15 Additionally, it was shown that Zika infected the iris, retina and optic nerve, leading to panuveitis and neuroretinitis in addition to conjunctivitis. These observations are reminiscent of another recent viral epidemic, the West African Ebola outbreak of 2014. Of course, Ebola is much different from Zika: It is a hemorrhagic fever virus spread without an insect vector, and it primarily impacts the gastrointestinal system, where it is often fatal without significant supportive care. Despite these differences, a well-documented case of an Ebola survivor reported that a convalescing patient presented with uveitis;16 subsequent to this, follow-up identified 57 Ebola survivors with uveitis, suggesting that infectious virus or viral RNA in the eye may have triggered this complication.17 It may be that some aspects of ocular physiology leave the eye susceptible to viral infiltration and/or retention, and thus any viral conjunctivitis may have this same risk.
The life cycle of viruses involves an intracellular growth and assembly phase and a cell lysis phase where newly-formed viruses are released to infect other cells. This type of life cycle means it’s more difficult to clear a viral infection once it has been introduced; some viruses lay dormant in a nonproliferative stage within the host cell, further extending their inhabitance. These host-pathogen interactions, along with the highly genetically diverse group of viruses that can induce conjunctivitis, have complicated the discovery of effective therapeutics for viral infections.
A promising new therapeutic approach to treatment of viral conjunctivitis is represented by OKG-0301 (Okogen, Encinitas, Calif.). While most antiviral drugs act by inhibition of nucleic acid biosynthesis, OKG-0301 is a ribonuclease that acts to inhibit viral replication by interfering with viral protein synthesis. OKG-0301 also interferes with the inflammatory response by inhibiting NF-κB, a transcription regulator that is a key signal point in the inflammation process. Another new product in development, APD-209 (Adenovir Pharma; Helsingborg, Sweden), is designed to treat epidemic keratoconjunctivitis by preventing adenoviral binding and entry into cells. These examples of new therapies are particularly exciting because they represent new mechanistic strategies in the design of antiviral drugs.
The silver lining of these recent viral epidemics is that they rekindle the experimental fires, giving a much-needed boost to research into new antiviral therapies. Hopefully this renewed interest will lead to more effective ways to treat and eliminate all viral conditions, including ocular viral infections.
Dr. Abelson is a clinical professor of ophthalmology at Harvard Medical School. Mr. Shapiro is vice president at the ophthalmic consulting firm Ora. Dr. Slocum is a medical writer at Ora. Dr. Hollander is chief medical officer at Ora, and assistant clinical professor of ophthalmology at the Jules Stein Eye Institute at the University of California, Los Angeles.
1. Rietveld RP, G. ter Riet, Bindels PJ et al. The treatment of acute infectious conjunctivitis with fusidic acid: A randomised controlled trial. Br J Gen Pract 2015;55:521:924-930.
2. Collin HB, Abelson MB. Herpes simplex virus in human cornea, retro-corneal fibrous membrane, and vitreous. Arch Ophthalmol. 1976 Oct;94:10:1726-9.
3. http://www.who.int/mediacentre/factsheets/fs382/en/
Accessed 2 Dec 2016.
4. Rose PW, Harnden A, Brueggemann AB, et al. Chloramphenicol treatment for acute infective conjunctivitis in children in primary care: a randomised double-blind placebo-controlled trial. Lancet. 2005;366:9479: 37-43.
5. Patel PB, Diaz MC, Bennett JE and Attia MW. Clinical features of bacterial conjunctivitis in children. Acad Emerg Med.2007; 14:1:1-5.
6. http://www.aoa.org/documents/CPG-11.pdf. accessed 18Nov2016
7. Azari AA and Barney NP. Conjunctivitis: A systematic review of diagnosis and treatment. JAMA 2013;310:16:1721-1729.
8. Jackson WB. Differentiating conjunctivitis of diverse origins. Surv Ophthalmol 1993;38:S91.
9. Morrow GL, Abbot RL. Conjunctivitis. AM Fam Physician 1998;57:4735-46.
10. Jhanji V, Chan TC, Li EY, Agarwal K, Vajpayee RB. Adenoviral Keratoconjunctivitis. Surv Ophthalmol. 2015; 60:5:435-43.
11. Kam KY, Ong HS, Bunce C, Ogunbowale L, Verma S. Sensitivity and specificity of the AdenoPlus point-of-care system in detecting adenovirus in conjunctivitis patients at an ophthalmic emergency department: A diagnostic accuracy study. Br J Ophthalmol. 2015; 99:9:1186-9.
12.https://www.aao.org/preferred-practice-pattern/conjunctivitis-ppp--2013 accessed 18 Nov 2016.
13. Newman H and Gooding C. Viral ocular manifestations: A broad overview. Rev Med Virol 2013;23:281-294.
14. Murray JS. Understanding Zika virus. J Spec Pediatr Nurs. 2016 Nov 9. [Epub ahead of print]
15. Miner JJ, Sene A, Richner J et al. Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Reports 2016;16:12:3208-3218.
16. Varkey JB, Shantha JG, Crozier I, et al. Persistence of Ebola Virus in Ocular Fluid during Convalescence. N Engl J Med 2015;372:2423–2427.
17. Tiffany A, Vetter P, Mattia J, et al. Ebola virus disease complications as experienced by survivors in Sierra Leone. Clin Infect Dis 2016;62:1360–1366.



