Placing an IOL in the sulcus instead of the capsular bag is rarely a surgeon’s preference, but sometimes intraoperative circumstances require it. In that situation, choosing the right lens and placing it properly can make the difference between a perfectly good outcome that will last for years and a potential future problem.
Here, experienced surgeons offer detailed advice on what to do, how to do it, and why it works.
When to Choose the Sulcus
“Putting the lens into the sulcus isn’t a first choice,” notes Uday Devgan, MD, FACS, FRCS, chief of ophthalmology at Olive View UCLA Medical Center and associate clinical professor at the UCLA School of Medicine. “The ideal is to put the lens in the capsular bag so that the natural bag that held the human lens will now hold the man-made lens. That’s what we do in 99 percent of eyes. It puts the IOL in the best, most stable, least risky and most physiologic position. We typically only place an IOL in the sulcus when there’s a defect in the posterior capsule that would prevent us from placing the IOL inside the bag.
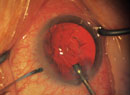
 |
| Because intraocular lenses available in the United States are not designed for sulcus placement, they’re often at risk of decentering without additional support. Above: a three-piece IOL in the sulcus, sunsetting following trauma to the eye. |
“Nevertheless, sulcus placement is more common than you might think,” he says. “In the United States, the average surgeon does 250 cataracts in a year, and somewhere between 1 and 5 percent of cataract cases result in a complication that would cause the surgeon to place the lens in the sulcus. Generally, sulcus placement happens in eyes that have a posterior capsule defect, which is the most common reason, or because the eye has loose zonules.”
Use of sulcus placement in the latter situation, however, is subject to some debate. “Some people have said if there’s zonular dehiscence or severe pseudoexfoliation, you might be better off putting a lens in the sulcus, possibly with a capsular tension ring underneath,” notes Douglas K. Grayson, MD, medical director and chief of glaucoma and cataract surgery at Omni Eye Services in New York and New Jersey, and assistant professor of ophthalmology at the New York Ear and Ear Infirmary of Mt. Sinai and the Hackensack Meridian School of Medicine. “The reasoning is that putting a lens in the bag may stress the zonules and cause them to break, resulting in the lens being likely to decenter at a higher rate in the future. I haven’t generally put the lens in the sulcus in that situation, because I feel that if I have enough zonules to get the lens into the bag in the first place it may be more stable that way, pushing out the zonules. However, I can see some merit to the other point of view, especially in cases of really severe pseudoexfoliation or zonular compromise due to trauma.”
Jorge L. Alio, MD, PhD, a professor and chairman of the department of ophthalmology at Miguel Hernandez University in Alicante, Spain, says he considers placing a lens in the sulcus when he encounters an unstable capsular bag. “If I have a subluxation, for example, I prefer the sulcus-placed lens, even though I also use a capsular tension ring to fix the capsular bag in place,” he says. “My experience is that even with the capsular tension ring, sometimes you’ll have a lens dislocation over time. I also prefer to use a sulcus-placed lens when piggybacking to deal with a refractive surprise, or in secondary implantations when I have to explant an IOL—for instance, following opacification. Of course, in these cases I’d rather put the lens into the capsular bag, if this is possible.”
“Some surgeons place a sulcus IOL in eyes with uveitis to avoid iris-capsule synechiae, but I disagree with that reasoning,” Dr. Devgan adds. “In that situation I just perform a large capsulorhexis.”
Picking the Right Lens
“Placing the lens in the sulcus is a great option when the posterior capsule can’t support an intraocular lens in the bag and the anterior capsule is intact,” says Thomas A. Oetting, MD, MS, a professor of clinical ophthalmology at the University of Iowa. “The problem in the United States, at least, is that we no longer have a perfect sulcus IOL. We only have three-piece IOLs with relatively short haptic lengths. As such, the only way to ensure long-term stability with sulcus haptic placement is to have an intact anterior capsule that allows capture of the optic into the bag.”
Dr. Oetting points out that none of the FDA-approved in-the-bag IOLs can be counted on to remain stable in the sulcus. “The main reason for this is that the haptics aren’t long enough,” he explains. “What you want is a haptic length of about 14 mm or more. The longest haptic length we have in the U.S. is 13 mm. If you simply place an IOL with a 13-mm haptic length in the sulcus of a large eye without capturing the optic, it can decenter and tilt.
“Pavlina S. Kemp, MD, from our group, conducted a study1 which showed that when the eyes are bigger, you can’t count on the Alcon MA50—which has a 13-mm haptic length—to stay centered long-term,” he notes. “That means that if you place an IOL in the sulcus, unless it’s a small eye, you should capture the optic with the anterior capsule. If you can’t do optic capture, the IOL may decenter over time. In the United States we used to have access to the STAAR AQ2010, that was well-suited for sulcus placement because it had a 14-mm haptic and a large optic, but that lens is no longer available to us. As a result, if you’re not able to capture the optic, you may be forced to place a lens in the sulcus knowing that it may decenter over time.”
To make the best of this situation, Dr. Oetting notes that you need to pick a three-piece lens with long, thin haptics.2 “You want the longest haptics you can get,” he says. “Also, you want a lens with a big optic, so that if it decenters a little bit it will be less of an issue.”
Whether a sulcus-based lens should be made of acrylic or silicone is subject to some debate. “In terms of material, I think silicone three-piece lenses do better in the sulcus than acrylic three-piece lenses, because acrylic lenses are more slippery, while the silicone is a little bit more adhesive,” says Dr. Grayson. “Whatever capsule is left will adhere better to the silicone, keeping the lens in a better position. One of the best lenses for sulcus placement was the STAAR AQ2010, which I don’t think you can get anymore. That was a silicone three-piece with a 6.5-mm optic and polyamide haptics, which were very stiff. The next-best lens is the Tecnis Z9002 silicone three-piece. It has rounded, not square, edges, so you’re not going to get as much chafing.”
Dr. Oetting, however, notes some reasons to choose a lens made of acrylic rather than silicone. “One of the issues you have to keep in mind is that when we’re placing these lenses in the sulcus, there’s often a posterior capsule injury,” he says. “Those patients are at increased risk of needing a vitrectomy in the future, and that may involve silicone oil. Silicone lenses can have problems with air-fluid exchanges and silicone oil; lenses made of acrylic won’t have a problem in that situation.
“The IOL I tend to use, which is a decent lens for the sulcus, is the Alcon MA50,” he adds. “It’s a three-piece lens with a 6.5-mm acrylic optic, which makes it suitable for capturing in slightly large anterior capsulotomies. However, this still isn’t a perfect choice, because the haptic length isn’t long enough.”
One-piece IOLs in the Sulcus
Dr. Devgan says that three-piece lenses work better in the sulcus than most one-piece lenses. “When you talk about single-piece lenses, you’re primarily talking about the single-piece acrylic lenses,” he notes. “You must never place a single-piece acrylic IOL in the sulcus. That’s an absolute rule—the single-piece acrylic IOLs are meant solely for in-the-bag placement. (Surgeons reiterate this in a response to this article in the September issue's Letters to the Editor: http://www.reviewofophthalmology.com/article/taking-issue-with-sulcus-iol-choices)
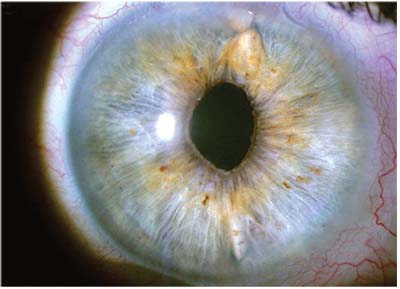 |
| One way to provide additional support for an IOL placed in the sulcus is to suture the haptics to the iris or sclera. Above: The eye shown on the previous page, with the IOL optic recentered and the haptics sutured to the iris at 12 and 6 o’clock. |
“There are three main problems with them in the sulcus,” he explains. “First, the haptics of a single-piece acrylic lens are as thick as the lens itself. A thick haptic can chronically rub the back side of the iris, causing the iris to lose pigment, leading to inflammation and microbleeding in the eye and causing UGH syndrome—uveitis, glaucoma and hyphema. The lens will also tend to decenter; then the surgeon will have to explant and exchange the lens. In comparison, the haptics of a three-piece lens are much thinner.
“For example, I saw one patient whose surgeon had placed a single-piece acrylic in the sulcus,” he says. “The thick haptic had been chronically rubbing raw the back side of the iris. It scraped the back of the iris so much that the red reflex, the iris transillumination defect, was literally the outline of the haptic. This is not what you want! So you should never put a single-piece acrylic lens in the sulcus.
“A second issue is that the lens of a single-piece IOL is planar,” he continues. “If you look at its side profile, it’s totally flat. In contrast, if you look at a three-piece lens from the side, the lens is slightly posteriorly vaulted. That means the optic is set back a little, so it doesn’t scrape the back of the iris.
“The third issue,” he adds, “is that the single-piece acrylic IOLs may be smaller in total length than the three-piece IOLs, and their haptics don’t have sufficient rigidity. As such, they don’t fit well in the sulcus. They tend to sunset.”
Dr. Devgan notes, however, that a single-piece lens made of PMMA can sometimes work in the sulcus. “The PMMA single-piece lenses are rigid and nonfoldable,” he explains. “Even though they’re single-piece, they have very thin arms, and they’re OK for sulcus placement. Not the best, but OK. However, those lenses are rarely used in the United States—they represent far less than 1 percent of the market. That’s because being rigid and nonfoldable, they require a 6- or 7-mm incision to put them inside the eye. Today, we make 2.5-mm incisions—or smaller—all day long.”
Dr. Grayson notes one situation in which he’d consider putting a one-piece IOL in the sulcus. “If your surgical plan was to put in a Symfony or a Symfony toric and you break the posterior capsule, you don’t have too many options,” he says. “You either try to place the lens in the sulcus with optic capture through the capsulorhexis, or you have to abandon that lens and put in a monofocal. Alternatively, you could put in a multifocal three-piece of a different design, such as the Alcon ReSTOR with the 4.0 add, or the Tecnis with the 4.0 add. If there was an astigmatic component, you’d have to deal with it later using PRK.
“The problem with changing to a different lens,” he notes, “is that the patient has paid $3,500 or $4,000 ahead of time and is expecting to get a certain result. It’s hard to just say, ‘Sorry, we couldn’t do it.’ It’s even worse if you’ve done one eye with the Symfony toric, and you’re on the second eye.
“Of course, without optic capture, the one-piece lenses are too small and too thick to place in the sulcus, and they move too much,” he says. “For that reason I wouldn’t put a one-piece in the sulcus unless I had anterior capsular capture, so the optic is in the bag, below the capsular edge. The haptics of a one-piece lens are thick, but the tension from the lens capture will keep them away from the iris. They won’t be sitting flat; they’ll be angled slightly downward. So there’s a very good chance that you’ll be able to do this, especially with a capsulorhexis made by a femtosecond laser, which I always use when implanting multifocals, premium lenses and torics. In addition, capturing the lens works out well—especially for torics—because they don’t move.”
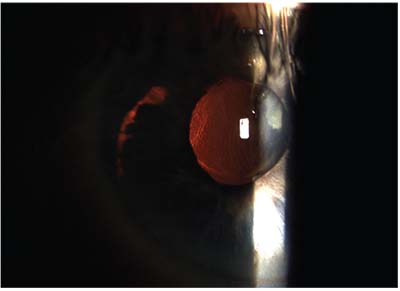 |
| Placing a single-piece IOL in the sulcus is never recommended. Above: a decentered single-piece acrylic multifocal IOL has decentered and is now causing damage to the back of the iris, as demonstrated by the transillumination defect in the shape of the IOL haptic. |
The Lens-capture Option
Dr. Devgan says that having the haptics in the sulcus with the optic captured back through the capsulorhexis is probably the best position for long-term IOL stability. “Doing that is like putting a manhole cover on a manhole,” he says. “The lens becomes a really good barrier, so you won’t get vitreous prolapse, and it’s really solid. It will never move. Furthermore, because you’re pushing the optic farther back, close to where it would have been if it were placed inside the bag, the issue of refractive shift is minimized. You don’t need to adjust the lens power.”
“Using IOL capture when putting a lens in the sulcus is by far the best way to do it, and femtosecond lasers have made that a lot easier because you get a perfectly symmetrical capsulotomy of a consistent size,” notes Dr. Grayson. “If you create a 5- or 4.8-mm capsulotomy with a femtosecond laser—I use 4.8 mm—you can then capture the optic. Using this approach you can put a three-piece lens in the sulcus; in fact, you can even put a one-piece lens in the sulcus. I’ve put Symfony torics in the sulcus using IOL capture after femtosecond capsulorhexis, and it’s worked out fine.”
Dr. Oetting agrees that when you need to place a three-piece IOL in the sulcus, the best approach is to use optic capture. “That’s clearly the best choice if you can’t place the IOL in the bag,” he says. “In fact, it may even be better than simply placing the IOL in the bag in cases where you’re worried about progressive zonular weakness, such as pseudoexfoliation and retinitis pigmentosa. By placing the haptics in the sulcus and the optic in the bag you nearly eliminate the possibility of phimosis. The anterior capsule can only contract so much because the lens is keeping it open. Because of that, you tend to get less zonular damage and more long-term stabilization. The haptics in the sulcus provide additional centration and support, independent of the zonules. I really think it’s a nice technique to have in your bag of tricks.”
Dr. Oetting also notes that zonular dehiscence can sometimes be a reason to consider placing the IOL haptics in the sulcus. “For example,” he says, “if you had mild zonular weakness and were worried about phimosis and long-term centration of the lens, it would be a good option to place a three-piece lens with the haptics in the sulcus and the optic in the bag. That can give you really nice centration both short and long term.
“However,” he adds, “you do have other options in that situation, including placing a capsular tension ring and then putting a standard lens in the bag, or placing an Ahmed segment in the area of weakness, and then putting the lens in the bag. So placing the lens in the sulcus is just one of several options when you’re dealing with weak zonules.”
Dr. Oetting points out another capture-based option that’s useful when you’re faced with a very young patient or someone who won’t be able to sit still for a possible future YAG capsulotomy, a technique he learned from Lisa Arbisser, MD. “In addition to creating an anterior capsule opening, you can also create a posterior capsule opening that’s round and continuous,” he explains. “Then you place the haptics in the sulcus and push the optic all the way back, so it’s captured by both the anterior and posterior capsule. Doing this is a two-for-one, because it creates a very stable lens configuration that dramatically reduces the possibility of lens decentration and also prevents posterior capsular opacity. Dr. Arbisser calls this ‘bicapsular capture,’ and while the indication doesn’t come up very often, the technique can be very useful.” Dr. Oetting adds that when using this technique an IOL can often be placed with no vitreous prolapse, despite the posterior capsular opening. “If vitreous prolapse does occur,” he says, “you’ll have to perform an anterior vitrectomy to limit vitreous traction and the risk of retinal detachment.”
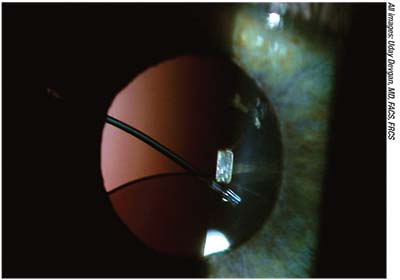
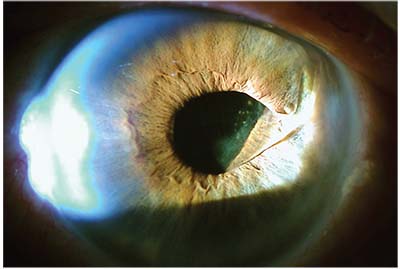 |
| A single-piece acrylic IOL loose in the sulcus with one of the haptic coming above the iris. |
Dr. Oetting says that he’s aware of some options for stabilizing a lens in the sulcus (when the optic isn’t captured) that he hasn’t tried. “Some people talk about bending the haptics to change the angle at which the haptic comes off of the lens, effectively making them longer,” he says. “Another option is to suture the lens to the iris. You could also try using the Yamane technique, or the Agarwal glued-IOL technique with a three-piece IOL. And it’s always an option to place an anterior chamber lens. But just placing the haptics into the sulcus in a large eye [without using any of these techniques] is inviting decentration.”
Getting the Lens In
As with any surgery, using the right technique matters. Surgeons offer these suggestions to minimize the likelihood of an undesirable outcome:
• Enlarge your incision slightly. “Most three-piece IOL inserters require a bigger incision,” notes Dr.
Oetting. “Focus on making it easy to get the injector in. Don’t try to force it or push it hard through a too-small incision. If you push hard, you may lose some viscoelastic, and the next thing you know, vitreous is coming forward.”
• Use a dispersive viscoelastic. “When you find you have an issue with the posterior capsule, you want to be smart about your choice of viscoelastic,” says Dr. Oetting. “I usually switch over to a dispersive viscous OVD like Alcon’s Viscoat. Use the OVD to create space between the iris and the anterior capsule to better allow the leading haptic into the sulcus. Ideally you want to open the sulcus for 360 degrees, although that can be hard to do.
“The reason a dispersive OVD is ideal is that in cases like this you’re very likely to leave a little bit of viscoelastic material behind,” he explains. “You’ll get fewer pressure spikes if the OVD you leave behind is dispersive rather than cohesive. A dispersive viscoelastic also coats and sticks to tissue better.”
• Insert the lens carefully. “My incision size is about 2.75 mm, as we need to introduce the tip of the injector into the anterior chamber,” says Dr. Alio, noting that he prefers to use the Alcon MA60 or MN60 lenses. “I deliver the distal haptic into the sulcus and place the trailing haptic over the iris. Then with a Lester hook I carefully dial the second haptic into the sulcus. Finally, I use a Sinskey hook to check for stability and see that the lens is properly placed and stable.”
“When you’re putting a lens in the sulcus, make sure the haptic is on top of the capsular shelf,” says Dr. Grayson. “You can skim the haptic along the bottom edge of the iris. Then, once you launch the lens, you’re usually left with one haptic outside the eye. You want to carefully rotate that in, skimming the back surface of the iris to make sure that the haptic stays in the sulcus. You don’t want to have one haptic end up stuffed into capsular bag remnants, and the other in the sulcus; then you’ll have uneven torsional forces which can cause the lens to decenter.”
“After you think the lens is in position, use your chopper to lift up the iris,” Dr. Devgan adds. “You can directly visualize whether the haptic is in the sulcus. It should be in front of the anterior capsular bag.”
• Place the haptics where the lens will get the most support. “If you have an area where there’s weakness, such as a defect in the posterior capsule and/or weak zonules, you don’t want to put the sulcus lens haptics where the weakness is,” says Dr. Devgan. “So, if the weak zonules are at 12:00, I put the lens horizontally at 3:00 and 9:00.” (He adds that if the anterior capsule rim is intact and no zonular weakness is present, the orientation of the lens doesn’t really matter.)
• Consider using a capsular tension ring. Dr. Grayson notes that in terms of allowing the lens to be placed in the bag, a capsular tension ring can’t always save the day. “Once a bag has been compromised, I don’t think you want to put a capsular tension ring in there,” he says. “It’s not going to go well. It will end up dragging the capsule and ripping more zonules. It can also be challenging just to get the CTR into the bag if the bag is compromised.”
Dr. Grayson says in certain rare circumstances, he might place a capsular tension ring in the sulcus with the lens on top of it. “That might be a more stable situation than in-the-bag placement,” he says. “Normally we use a capsular tension ring when we have zonular compromise with an intact bag. But if you don’t have an intact bag, or if you have an intact bag with bad pseudoexfoliation and you don’t want to put the lens in the bag, putting the ring in the bag isn’t going to help. Putting a capsular tension ring in the sulcus is not a common thing to do, and I’ve only done it once or twice, but it may provide more of a shelf to keep the haptics in the sulcus.”
• Avoid sulcus placement if the eye isn’t stable. “If things don’t look stable, you shouldn’t put the lens in the sulcus,” says Dr. Grayson. “It’s important to make sure the eye has enough of an anterior capsular shelf to allow sulcus placement. If half of the anterior capsular shelf is gone, and your posterior capsule is gone, the sulcus may not be a stable location. This is a situation that requires a judgment call. If the eye is only missing 90 degrees of the anterior capsular shelf, odds are that the lens will be stable enough in the sulcus. It might even be possible to place a capsular tension ring in the sulcus and place the lens on top of it. But if you have 180 degrees of support missing, even with a capsular tension ring, anything in there could take a dive.”
Should You Suture?
Dr. Devgan says he doesn’t usually use suture fixation unless the lens is clearly not stable. “If there’s insufficient support in the sulcus, such as loss of zonules or lack of an anterior capsular rim, then sutures may be needed to fixate the sulcus IOL to the back of the iris or to the sclera,” he explains, noting that this can happen postoperatively as well. “For example, a patient of mine had a sulcus lens that looked great for a long time. Then the patient suffered trauma and came to see me, and I discovered that the lens was slipping. To make sure the lens never moved again,
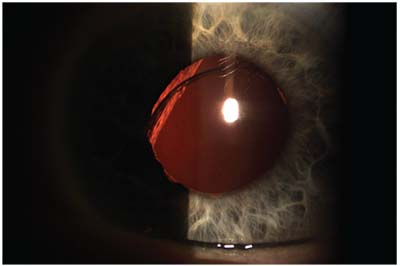 |
| A single-piece acrylic IOL sunsetting in the visual axis/pupil zone. |
I put two stitches in his iris, one at 6:00, one at 12:00. (See pictures on p. 23 and 24.) Those are permanent stitches that are meant to be there forever. In the ‘after’ picture, you can even see the blue stitches.”
Dr. Alio says he would resort to suturing a sulcus lens in place if he didn’t find good stability, there was vitreous loss and, especially, if there was some question about capsular support. “If there’s less than 50 percent of the capsule left, I prefer to suture the lens to the iris or to the sulcus,” he says. “If I see that 50 percent or more of the capsular bag still exists, then I place the haptics in a way that will bring the lens to a stable position. If there’s any doubt about this, I suture the haptic that’s positioned in the not-well-supported area to the iris.”
Dr. Grayson notes that suturing a lens in the sulcus can be problematic, even if the suturing goes well. “When we’ve sutured lenses into the sulcus, the lenses would sometimes torque,” he says. “This has sometimes resulted in cystoid macular edema, and we couldn’t isolate the source of the CME because we didn’t know exactly where the haptic was digging in. So sutured sulcus IOLs are not problem-free. A nice anterior chamber lens that’s well-positioned and sized does just fine, and is probably a better option than a sutured sulcus IOL.”
In some situations, a surgeon might feel pressured to use a one-piece lens in the sulcus (despite the drawbacks of doing so), and try to suture it in place. Dr. Grayson notes that suturing a one-piece lens in the sulcus should never be a surgeon’s first choice. “If you suture a three-piece lens, the incidence of iris chafing and secondary pigment glaucoma is very low,” he notes. “It’s much higher with a one-piece, so I don’t think you should suture a one-piece to the iris unless you absolutely have to.
“Of course,” he continues, “plenty of surgeons will argue that this should never be done, no matter what the circumstances are. But there are some situations in which it’s the best option, especially if it’s done correctly. For example, there might already be a one-piece lens in the eye, and it’s only slightly decentered. I have patients that I placed a ReSTOR into 15 years ago and now the lens is just slightly decentered due to pseudoexfoliation. I can bring that lens back into position, suture it loosely to the iris, and the patient still gets the total functionality of the lens. But you have to use relatively loose sutures; if you tie it to the iris too tightly, you’ll produce iris chafing.
“However,” he adds, “when you’re planning a procedure in an aphakic eye where you know you’ll be suturing the lens to the iris, I definitely would not choose a one-piece lens.”
Dr. Grayson adds that he advises not suturing the lens in the sulcus if the surgery is already problematic. “I don’t like suturing the lens into the sulcus in the face of a crisis,” he says. “When the phaco has gone poorly, and you’ve blown away enough of the capsule that suturing becomes the only way to support the lens, you have to evaluate where you are. For example, was the patient blocked ahead of time or did you use topical anesthesia? That’s an issue because patients can only tolerate so much time on the table with topical anesthesia, and suturing a lens, even for the pros, is time-intensive. Speakers on the podium often make suturing sound like a trivial thing. It’s not trivial! I’m pretty good at it, and it’s still labor-intensive. So I don’t think it’s a good idea to do this in the midst of a crisis.
“Ideally, suturing the lens in the sulcus should be a planned procedure,” he notes. “You don’t want to have a patient who signed up for a routine cataract surgery lying there for an hour and a half on the table. You’ll start to lose control, and then you’ll get messy results. If you can’t put a lens in the sulcus without suturing it, then your best options are to either leave the patient aphakic for the time being, or put in an anterior chamber lens.
“Nobody should be afraid of just leaving the eye without a lens,” he adds. “You can come back another day and put in a sulcus- or iris-fixated IOL. If the surgery up to that point has been difficult, leaving the eye alone and coming back another day gives the cornea a chance to clear. If it doesn’t clear and the patient ends up needing a DSEK, at least that can be a nice, planned procedure with a sutured lens. Surgeons shouldn’t feel this intense pressure to implant a lens in a crisis situation. Those are the cases that end up doing the worst.
“And remember,” he adds, “there’s nothing wrong with placing a good anterior chamber lens, as long as the lens fits and it’s well-positioned.”
Adjusting the Lens Power
Moving the lens forward inside the eye—and possibly changing the type of lens—may necessitate adjusting the refractive power of the lens. Surgeons have different preferred approaches to deciding how much refractive adjustment is necessary.
“If you can’t use lens capture, you have to go a solid half-diopter lower,” says Dr. Grayson. “You also have to take into consideration the A-constant, because most of the one-piece lenses are aspheric and have a relatively high A-constant, in contrast to most of the three-piece lenses, which are plano convex, giving them a lower A-constant. That means those lenses are already a half-diopter lower.
“For example,” he continues, “if you planned to use a one-piece 20-D lens, but you’re going to the sulcus and switching to a three-piece, you’d probably go a half-diopter lower to compensate for the different position, but you’d have to go another half-diopter down because of the A-constant. In effect, you’re going down a full diopter. If you’re able to do an IOL capture, then you could probably just go down a half-diopter and use the 19.5-D three-piece. You have to make that calculation in your head on the fly, unless you write it out ahead of time, which would be ideal.” (Dr. Grayson adds that if you can capture the lens in the anterior capsule, you may not need to change the refractive power at all.)
Dr. Devgan has created a simple system that tells him how much he’ll need to adjust the lens power if he finds that the lens needs to be placed in the sulcus instead of the bag; he calls it the rule of nines. “The first step is to make sure your A-constant is accounted for,” he says. “All lenses are slightly different, even if they’re marked the same power. A 20-D lens, for example, may not be the same as a 20-D lens from a different manufacturer. The A-constant allows us to compensate for factors such as lens geometry, the lens material and how much posterior vault the lens has. It’s a number you need when you do your lens-power calculation, and it’s specific to every type of lens.
“For example, if a surgeon typically uses a single-piece acrylic IOL where the A-constant is 119.2 when the lens is in the bag, he or she must begin by calculating the power for the three-piece IOL that will be placed in the sulcus, where the A-constant is 118.7 when the lens is in the bag,” he says. “That means dropping the IOL power by 0.5 diopters, which is the difference between 119.2 and 118.7. Of course, if you just use the same three-piece lens for every patient, then this is a moot point.
“Once that’s accounted for, you can use the rule of nines,” he continues. “That will tell you how much you need to lower the lens power. In a nutshell, if the lens power is between 0 and 9 D, you don’t need to change the power when you move it forward. If it’s between 9.5 and 18 D, you subtract 0.5 D from the power. If it’s between 18.5 and 27 D, you subtract 1 D. If it’s above 27 D, subtract 1.5 D. And so forth.
“For example,” he continues, “let’s say a patient is so myopic that the lens power is zero. That means the lens is plano; it has no power at all. In this case, it doesn’t matter where you put the lens. This is basically true as long as the lens power is below 9 D; moving it doesn’t cause much of a change in its power. On the other hand, a 30-D lens is powerful; moving it just a little will change the power a lot. So you have to subtract 1.5 D from the power if you move it forward from the bag to the sulcus.”
Dr. Devgan notes that the rule of nines is an estimation. “Of course you can calculate the exact amount of change for the lens you’re dealing with if you want to, but it comes out to be the same, because lenses only come in half-diopter steps,” he says. “A 12-D and 18-D lens are not the same, but since the lenses only come in half steps, the amount of change needed when you move it forward into the sulcus is about a half-diopter less for both lenses.”
Strategies for Success
Surgeons offer these general suggestions to help ensure that you won’t be caught off-guard when the need to place a lens in the sulcus suddenly arises:
• Have an appropriate lens available. Dr. Oetting says he believes you shouldn’t start surgery unless you have a lens for the sulcus at hand. “This is one situation that surgeons are likely to encounter from time to time,” he says. “You’re working at a surgery center and all they have for you to work with is a single-piece lens. If you run into trouble, it forces you to think about placing a single-piece lens in the sulcus—a bad idea—or putting the single-piece lens into a compromised bag. Either way you’re setting yourself up for potential trouble.
“I think no matter where you’re operating, you have to have an anterior chamber IOL and a sulcus lens available,” he continues. “Is it likely that you’ll need to place a lens in the sulcus? No. Could you stop and bring the patient to another OR, or have them come back another day to put a lens in? Yes. But to me it seems like the better thing to do is be prepared and have a consignment of sulcus lenses and a consignment of anterior chamber lenses wherever you operate.”
• Practice using the three-piece injector that goes with your preferred sulcus IOL ahead of time. “All of the three-piece injectors are slightly different,” Dr. Oetting points out. “So, if you’re going to use a particular sulcus lens, the time to practice using that injector is not when you’re in the middle of a horrible case. Practice using that injector when the seas are calm.
“I suggest placing your choice of three-piece lens in a few standard cases,” he continues. “That will help you get used to putting it in. You can go over this with your company rep a few times. Another option is to practice on a Phillips artificial eye; you can practice making an anterior capsulotomy and placing lenses in the sulcus or the bag. The point is to be accustomed to using the sulcus lens you’re going to choose. When you’re facing a difficult case, you should already know how to do what you need to do.”
Dr. Grayson points out that many surgeons today have become accustomed to using one-piece lenses and their injectors. “When they suddenly have to use a three-piece injector, it’s awkward,” he says. “For one thing, it’s loaded differently, so if your technician isn’t familiar with loading a three-piece, the haptics could end up damaged. Second, when you’re introducing it into the eye, the stiff haptics have a different feel. You have to make sure the haptics don’t scrape against the cornea, and you need to make sure you don’t introduce the lens with the haptic directly into the posterior cavity where it could potentially sink.
“Injecting a three-piece lens requires a certain amount of finesse,” he continues. “Any surgeon who’s been in practice for 25 years knows about this, because we didn’t have one-piece lenses for the longest time. But the younger surgeons are not accustomed to it. I see this when I do resident grand rounds. When they have to put in a three-piece, it’s a project because they’re not as familiar with it. Ideally, residents should be learning to implant both types of lenses.”
• Tell the patient what happened. “The reason this is a good idea is that eventually the different lens location will be noticed,” says Dr. Oetting. “Telling the patient right away is much better than saying nothing and having them be surprised later. I’ve had many patients come to me saying their doctor never told them that there was an issue when the lens was implanted, and they’re very disappointed in the surgeon. For that reason, I find it’s better to go through the discomfort of telling the patient that the surgery wasn’t perfect.
“Every patient understands that there can be issues during surgery,” he continues. “They just expect your expertise to carry them through so they don’t get into trouble. So, I tell the patient that we had a problem with the posterior capsule (‘It’s thinner than a red blood cell’) so I placed a different kind of lens. I tell these patients that they’ll likely do very well, but that I might watch them a little more closely because the IOL is in a different position inside the eye. I assure them they’ll have good vision with the new lens, and I reassure them again at the one-month visit.”
• Don’t co-manage these patients. “I don’t think this is a good situation in which to turn the patient over to somebody else,” says Dr. Oetting. “You should personally follow these patients closely. Make sure there’s no unusual inflammation and no increased intraocular pressure. Make sure the lens is staying centered. It’s not that big a deal, but it’s not a standard situation, so you should watch it a little more closely.”
• Take advantage of educational resources. Dr. Devgan says he recently launched a new website called
cataractcoach.com. “It’s totally noncommercial,” he says. “It simply coaches the viewer through difficult cataract surgery cases. I’ve been putting up new cases every day, recorded in HD with a full voiceover. One of them, for example, discusses dealing with a ruptured posterior capsule. It shows how to get the lens into the sulcus and make sure it’s secure. Another shows how to capture the optic of a three-piece lens in the sulcus back through the capsulorhexis.” Dr. Devgan says he’s planning to post a couple of new surgical videos every week.
• Be prepared—this will happen sooner or later. “An unexpected problem requiring IOL placement in the sulcus is the kind of thing that gets a surgeon’s heart racing,” notes Dr. Oetting. “Some surgical challenges happen with advance warning, so you can brush up on a technique before you use it, but sulcus IOL placement often comes up right in the middle of a routine case. This is something you simply have to be prepared for ahead of time. You may go for two years without encountering it, but then it happens.” REVIEW
Drs. Oetting, Grayson and Devgan report no financial ties to any product discussed. Dr. Alio is a consultant for Akkolens, Hanita Lenses, Oculentis and Zeiss.
1. Kemp PS, Oetting TA. Stability and safety of MA50 intraocular lens placed in the sulcus. Eye (Lond) 2015;29:11:1438-41.
2. Chang DF, Masket S, Miller KM, et al. Complications of sulcus placement of single-piece acrylic intraocular lenses: Recommendations for backup IOL implantation following posterior capsule rupture. ASCRS Cataract Clinical Committee. J Cataract Refract Surg 2009;35:8:1445-58.