For many decades, surgeons lowered IOP by circumventing the abnormal resistance of the eye’s inherent drainage system by performing trabeculectomy and creating a new outlet. This surgeon-made artificial outflow track abandoned the eye’s natural drain by shunting aqueous to the subconjunctival space, forming a bleb. The potential problems associated with bleb-forming surgery are well known to all ophthalmologists; they include hypotony maculopathy; scarring; dysesthesia; leaks; blebitis; and choroidal hemorrhage.
In recent years, thanks to advances in technology and the relentless pursuit of a better procedure, glaucoma surgeons are shifting away from bleb-forming procedures and moving toward more physiologic operations that enhance flow through the eye’s existing drainage system. Instead of abandoning the compromised outflow system, the surgeon and patient choose to try and enhance flow through the native collector system. This bleb-less option is used especially for patients with mild to moderate disease, before their natural collector channels have collapsed or atrophied.
The beauty of this type of micro-invasive canal-based surgery is that it improves flow into the patient’s own natural drainage system, instead of creating an artificial one. (When explained appropriately, most patients understand the concept of improving flow into their natural drain that’s been damaged by glaucoma.) This elegant microinvasive surgery also combines well with cataract surgery, making it possible to achieve two goals with one surgery, without substantially increasing the surgical risk.
Creating a limbal fistula to drain aqueous, as when implanting a filter, typically lowers IOP more than a canal-based procedure, but not every glaucoma patient has advanced disease that requires a subnormal IOP. A significant number of patients could have their glaucoma stabilized if their IOP could be lowered to the level of their episcleral venous pressure.
Despite the advantages of canal surgery, it has a few drawbacks. One major challenge is that it’s difficult to predict the success of the procedure. Here, we describe a strategy we’ve developed that can be used during the surgery to give the surgeon a sense of whether or not the minimally invasive glaucoma procedure is likely to lead to a favorable outcome.
Collector Channels Revisited
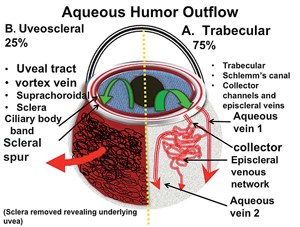
There are two major aqueous outflow pathways in the eye: conventional and nonconventional. Conventional outflow is the trabecular meshwork-Schlemm’s canal-collector channel path that empties into the episcleral veins; nonconventional outflow is via the uveoscleral pathway (i.e., suprachoroidal). Understanding the anatomy of these outflow pathways is key to understanding how the newer glaucoma surgeries work (See Figure 1).
|
Many surgeons are not well-acquainted with the conventional collector channels, probably for a couple of reasons. For one thing, you can’t easily visualize the collectors at the slit lamp during a clinical exam. Also, because canal surgery hasn’t been a common approach to treating glaucoma for many decades, there has been less need to pay attention to the collector channels. In the 1960s, the advent of trabeculotomy created excitement about angle surgery and spurred extensive research and discussion. However, segmental trabeculotomy with a metal trabeculotome, as invented by H. Mermann Burian, MD, and Lee Allen, MD, didn’t produce favorable long-term outcomes, so interest and excitement waned. The technology simply wasn’t as good as what we have today.
Ultimately, interest in the natural drainage channels plummeted when trabeculectomy was invented. This new surgery allowed surgeons to make a hole in the eye and create a new, nonphysiologic drainage channel, abandoning the patient’s natural collectors. After creating an external new drainage pathway, it was not as important to focus on the episcleral veins or collector channels. As a result, much of the science relating to the collector channels ground to a standstill, and their role in glaucoma therapy was slowly de-emphasized and eventually forgotten.
Today, however, canal-based surgeries are experiencing a renaissance. As a result, the collector channels have become a topic of interest again. However, our understanding of Schlemm’s canal, the distal collector system and wound healing in the angle lag far behind our understanding of trabeculectomy, glaucoma drainage tubes and wound modulation with anti-metabolites. Hopefully, this new focus on minimally invasive glaucoma surgery will provide greater insight into the canal and distal collector systems.
What Can Go Wrong
The purpose of canal surgery is to optimize the traditional conventional outflow pathway by shunting fluid into the collector channels and out through the episcleral veins. One problem with this approach has been that it’s very difficult to assess the preoperative, intraoperative or postoperative condition of the collector channels and episcleral veins. (For example, Matthias C. Grieshaber, MD, evaluated the collector channels with dye during canaloplasty and found that the degree of dye dispersion into the veins correlated with a lower postoperative IOP.1) Generally, if the surgery fails to produce the desired outcome, we’ve had to resort to guessing what the reason might be.
In contrast, with trabeculectomy there is a visible outcome marker: the bleb, which enables the surgeon to better understand why the procedure was successful or failed. If one visualizes a diffuse bleb and it correlates with a low IOP, it makes sense. If the IOP is elevated and a bleb is not present, one understands why the procedure failed. With canal-based surgery, the only marker we have is the postoperative pressure; if the pressure is low, we assume the collectors are working, but we don’t have any way to know for sure. That’s especially true when we do phacoemulsification at the same time, because it’s conceivable that the lowered pressure was a consequence of the cataract surgery.
If canal surgery fails, granted the above limitations, there are several possible reasons:
• The stent you placed didn’t end up next to a functioning collector channel. Unfortunately, the flow of aqueous around Schlemm’s canal is limited. That means we can’t expect to lower IOP simply by getting more fluid into the canal; we have to get it into the canal at points where there are nearby viable collector channels. That’s still a challenge because we don’t have any easy or definitive way to determine the location of the collectors.
• The collector channels have atrophied. One of the things that can go wrong in the eye’s natural drainage system is loss of the collector channels. This might happen, for example, if ongoing high IOP caused the trabecular meshwork to be constantly pushed against the back wall of Schlemm’s canal where the collectors are located, shutting them off, resulting in downstream channel atrophy. If you were to place an iStent next to atrophied collector channels, the result would likely be disappointing. Even if the surgery itself went perfectly, the fluid would have nowhere to go and the IOP would remain elevated.
In an ideal world, we’d have some way to evaluate the patency and capacity of the collector channels before deciding to do canal surgery, but at the moment no such method exists.
• The stent is malpositioned. There is a learning curve for all ophthalmic procedures. After insertion of a device, it may be malpositioned and therefore not functioning correctly. However, it can be difficult to determine whether the device is properly placed.
• Scarring has blocked the flow. If the IOP drops after the surgery and then climbs back up a few weeks or months later, the issue is most likely wound healing. Unfortunately, the reality is that we’re not able to modulate wound healing in the canal at this point in time. This is partly because we can’t see what’s happening; we don’t know if the collectors are closing down. And it’s not necessarily a simple thing to do; it took about 60 years to understand how to modulate wound healing with trabeculectomy so it didn’t scar down—and in some cases it still does anyway. We’re in our infancy trying to understand wound healing in the canal and collector channels, and that puts us at a disadvantage.
The overall problem for us as surgeons is that all of these issues are difficult, if not impossible, to evaluate. That’s where the episcleral venous fluid wave comes in; observing this wave near the end of surgery may provide real information about whether the device is properly located (or the Trabectome has been performed correctly) and the anatomic condition of nearby accessed collector channels, via the clock hours and the extent of the wave. The characteristics of the episcleral fluid wave may help predict the outcome of the procedure, and may suggest the reason for success or failure.
Collector System Anatomy
The human eye has 25 to 35 circumferential collector channels. Approximately six of these collectors are connected to large venous emissaries: the aqueous veins of Ascher. The majority of these larger veins are located nasally and inferiorly, especially inferonasally. That location is fortuitous, because most canal procedures are performed in the nasal quadrant using a temporal approach.
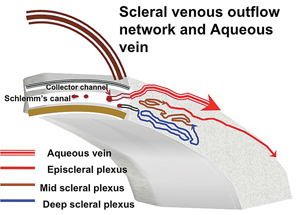
The collector channel system is intricate and difficult to evaluate. It runs through what is called the scleral plexus, which is fairly complex and contains several layers that aqueous must pass through to reach the episcleral veins; the exception is a major vein of Ascher, which has a direct route from the canal to the episcleral vein (See Figure 2).
|
One exception to this is the previously mentioned aqueous veins of Ascher, larger drainage collector channels first discovered in the 1950s by Norman Ascher, MD. While it’s not possible to see aqueous moving through most veins in the sclera, it is possible to see aqueous mixing with blood in vivo at the slit lamp in the larger aqueous veins of Ascher. And if you were able to place a stent next to one of these channels, it’s likely the surgery would produce more favorable results.
The Fluid Wave Explained
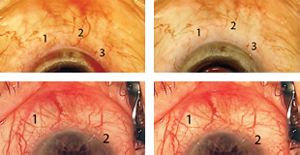
The episcleral venous fluid wave is a transient blanching of the episcleral vessels adjacent to a canal-based surgical site, seen during irrigation and aspiration, first described by us during a phacotrabectome surgery.2
Creation of the episcleral venous fluid wave is all about generating pressure differentials. Normally, the pressure in the episcleral veins is around 15 mmHg. If one stops the infusion of balanced salt solution during irrigation and aspiration, the intracameral pressure drops to about 2 or 3 mmHg. The resulting pressure differential encourages blood to reflux from the collector system into the anterior chamber through the canal, specifically in areas where the trabecular meshwork has been bypassed or ablated. If the infusion of BSS is then restarted, there’s a rapid reversal of the pressure gradient; BSS will flow out through the path of least resistance, which is usually the collector channels underlying the area of trabecular meshwork that has been cleaved or stented. This surge of BSS washes the blood into the downstream collector system and creates a blanching of the episcleral venous plexus (See Figure 3). When the veins fill with BSS, they either become invisible, or one is able to appreciate a train-track appearance of blood lining the inner-wall of the episcleral veins, representing laminar flow.
This visual change in the presence of the episcleral venous fluid wave tells the surgeon that there is communication between the anterior chamber and the downstream collector system. That means the collector channels are probably patent, even at the levels we cannot see; after all, the fluid has to go through those levels to make it to the visible veins. A good visible fluid wave is a strong piece of evidence that the collector channel system is at least anatomically functional, although there is no evidence of its physiologic function. (Of course, if you tried to generate the wave during a phacoemulsification procedure without canal surgery, you wouldn’t see anything, because an intact canal will not let BSS into the collector channels. The exchange of fluids in the veins doesn’t happen unless you’ve stented or opened the canal to the anterior chamber.)
Using the episcleral venous fluid wave to evaluate the condition of the collector system first occurred to us during viscocanalostomy. We noted that during viscocanalostomy, in which you unroof the canal ab externo, it was possible to inject BSS into the canal and see flow into the nearby episcleral veins. That was very exciting, because for the first time we had intraoperative evidence of the patency of the conventional collector system. Sometimes we would see a great deal of flow, sometimes minimal flow. The same thing happened with canaloplasty. When the Trabectome came along, we wondered if we could see the same phenomenon ab interno, a more self-contained model for evaluating episcleral flow, much like during the famed outflow cadaver studies of Paul Chandler and W. Morton Grant. Sure enough, we did. We quickly discovered that when you open up a section of the canal with the Trabectome you typically see the episcleral venous fluid wave at those same clock hours.2
This has been true for all the canal surgeries we’ve tried. If we implanted an iStent, we’d see one to two clock hours of flow into the adjacent veins. If we implanted a Hydrus shunt, which covers three to four clock hours of the canal, we’d see an equivalent area of flow. We recently published a technique we developed called gonioscopy-assisted transluminal trabeculotomy, or GATT, in which we open up 360 degrees of the canal using a microcatheter.3 This resulted in seeing the flow all the way around the sclera.
Of course, this is only true under optimal circumstances. There are cases in which the flow through the collector channels is minimal.
|
One likely reason the wave has not been appreciated until recently is that when we’re operating we’re always looking at what’s going on through the pupil or in the anterior chamber. During cataract surgery you’re not looking at the sclera. To observe the presence or absence of an episcleral venous fluid wave, you not only have to perform the test, you also have to make a point of looking at the adjacent episclera.
Real-World Use
We now use the fluid wave to test the patency of the collector channels as part of every canal procedure. Currently, for example, we frequently combine a Trabectome procedure with phacoemulsification. We start the procedure with the Trabectome, then do the cataract surgery. During the irrigation/aspiration portion, while removing viscoelastic, assuming everything is coming along nicely, the limbus and sclera are visualized to see the veins.
Next, we carry out the maneuver to elicit the episcleral venous fluid wave. We go to foot position zero and let the pressure drop, hopefully producing a reflux of blood from the veins. (The reflux spot is the best location to initially look for the wave.) Then we access foot position two, creating a surge of fluid into the anterior chamber. (We raise the BSS bottle height for this part.) The degree, location and extent of the wave are recorded. This adds a couple of minutes to the case. (If you need to, you can elicit the wave multiple times.)
If the flow is four-plus, an entire section of the sclera may blanch during the wave, not just the larger veins. You’ll often see a network of tiny veins between the larger veins, which have a reticulated network appearance. If the flow is good, the fluid will also push the blood down these veins, making the whole area briefly turn white. Of course, if your surgery opens the path into one of the three to six veins of Ascher, which likely connect directly to the canal, you may see a large surge of BSS into the vein of Ascher.
We think a four-plus wave is a sign that the patient is going to do well—or at least better than a patient who doesn’t have a wave. (We’re currently evaluating this.) Seeing a surge of fluid running down those veins means they are anatomically patent. Unfortunately, we can’t say with certainty that they’re functional, because functional means the aqueous will flow through there under normal physiologic conditions. Forcing fluid through them in the OR is not very physiologic, but it’s all we have. We can’t see the collectors preoperatively or postoperatively with any regularity, but by using the episcleral venous fluid wave we can at least see them in the operating room.
Riding the Wave
As canal surgery becomes more popular, the episcleral venous fluid wave can help us identify potential problems and give us a sense of whether the surgery is likely to be successful. It provides some information about the patency of the collector channels before we complete the surgery.
In addition, seeing the wave (or not seeing it) can also help the surgeon set appropriate patient expectations. You can tell the patient that you saw a lot of flow going through his collector channels, and that’s a good sign; or you might say you didn’t see a lot of flow, so you don’t know whether or not the pressure-lowering part of the procedure will work.
We think one day we’ll have an optical coherence tomographer or some other technology that will allow us to visualize and evaluate the collector channels. When we have that, we’ll be able to improve our results with canal-based surgery. We’ll be able to tell a patient that her collector channels can be salvaged. Or, we’ll be able to say that the patient’s distal collectors are atrophic and probably not functional, so we’ll do some other procedure, perhaps designed to increase flow into the uveoscleral pathway. If that doesn’t work, we’ll still be able to fall back on trabeculectomy. Unfortunately, the technology that will let us evaluate the collector system is probably five or 10 years away.
Microinvasive canal surgery is in its infancy, especially compared to trabeculectomy. Over time we’ll work out the best way to increase flow into the canal and the best way to modulate wound healing. It took a long time to improve trabeculectomy, and it will take a long time to improve canal surgery. But we think the odds of us improving canal surgery are much greater today because of our technology—and because small-incision cataract surgery works so well in conjunction with this type of canal procedure. REVIEW
Drs. Fellman and Grover are attending surgeons and clinicians at Glaucoma Associates of Texas in Dallas.
1. Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Clinical evaluation of the aqueous outflow system in primary open-angle glaucoma for canaloplasty. Invest Ophthalmol Vis Sci 2010;51:3:1498-504. doi: 10.1167/iovs.09-4327. Epub 2009 Nov 20.
2. Fellman RL, Grover DS. Episcleral Venous Fluid Wave: Intraoperative Evidence for Patency of the Conventional Outflow System. J Glaucoma 2012 Dec 31. [Epub ahead of print]
3. Grover DS, Godfrey DG, Smith O, Feuer WJ, Montes de Oca I, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: Technique report and preliminary results. Ophthalmology 2014;121:4:855-61. doi: 10.1016/j.ophtha.2013.11.001. Epub 2014 Jan 10.