When the term blebitis is used, it usually refers to delayed-onset in-fection of the bleb. Studies suggest that the rate of blebitis is about 0.1 percent long-term; although if you wear contact lenses, it increases by a factor of four.1 That’s why we generally don’t recommend wearing contact lenses—especially soft lenses—after a trabeculectomy.
Risk Factors for Blebitis
In order to minimize the chance of blebitis occurring, it helps to understand the factors that lead to it. Unfortunately, our understanding is far from complete. For example, it’s rare to have a bleb-associated infection without also having a bleb leak, so it seems sensible to assume that the leak comes first, allowing bacteria to enter the bleb. However, when you finally clear the infection, the leak usually goes away. That raises the possibility that the infection causes the leak—not the other way around. In truth, nobody really knows, because the two variables happen more or less concurrently.
Despite our lack of certainty about causes, we want to do everything possible during the surgery to reduce the likelihood that blebitis will occur. We know that several factors may play a role in increasing or decreasing the risk of blebitis:
• Use of antimetabolites. Anti-metabolites increase the risk of creat- ing a cystic, avascular bleb that’s more susceptible to infection. However, the use of antimetabolites is common because antimetabolites greatly en-hance the success rate of surgery.
One way to mitigate risk is to alter the way the antimetabolite is applied during surgery. In the past, we would often soak an absorbent material with the antimetabolite, place it on the sclera, cover it with the conjunctiva and leave it there. Eventually, a possible association between this technique and a domed, cystic, avascular bleb that was under tension, with a “ring of steel” around it—a bleb that was more likely to develop blebitis and endophthalmitis later—became apparent. (The avascular, cystic part of the bleb often correlated with the exact location of the mitomycin placement.) That approach has fallen out of favor; today, most surgeons take the absorbent material that’s soaked with the anti-fibrotic and place it over a broad area.
• Surgeon technique. Creating a low, diffuse, slightly vascularized bleb is considered ideal. Some literature by Peng Khaw, MD, at Moorfield’s Eye Hospital suggests that surgeon technique can impact this.2-4 Specifically, he suggests creating a fornix-based flap and trying to direct the flow from your trabeculectomy flap more posteriorly; he says this helps to create a slightly vascularized, low bleb.
• Location of the bleb. If you place the bleb inferiorly, or anywhere that it can be exposed, such as the interpalpebral space, the risk of infection goes up between ten- and twelvefold. The reason surgeons sometimes used to place trabeculectomies in those areas was that tube shunts weren’t a viable option yet; they still had high rates of complications and we weren’t sure how to use them effectively. So, when a trabeculectomy failed, we’d try to do another trabeculectomy. But when performing repeat trabeculectomies you quickly run out of real estate in the superior area, so surgeons moved inferiorly. This seemed quite logical at the time—but it was later realized that inferiorly located blebs had a higher risk of infection. (No one has conclusively demonstrated the reason for this.)
Of course, today, tube shunts and our ability to use them have improved, so the need to do multiple trabeculectomies has diminished. Now it’s common to do one trabeculectomy and then go straight to a tube shunt if the trabeculectomy fails.
Once the Patient Has Symptoms
The first priority when blebitis may be occurring is to make sure the patient realizes the danger and takes action as soon as symptoms are noticed. If blebitis is caught early enough and the bacteria that’s responsible is indolent, patients can do quite well. But if the patient delays seeking care, or it’s a virulent bacterium, then the blebitis can turn into endophthalmitis.
For that reason, it’s crucial to educate the patient. Clinicians typically ask the patient to be on the lookout for any new or unusual redness, pain or worsening of vision. We say, “If you have any of these—especially if you have all three—let us know right away, 24 hours a day, seven days a week, 365 days a year.” Every trabeculectomy patient needs to be aware of the dangers of long-term infection.
Once a patient contacts us to say that one or all of these symptoms are occurring, we insist he come in right away. Some physicians—a significant minority—even prescribe antibiotics for patients to keep at home, with instructions to begin using the drops and call in as soon as the patient notes the symptoms.
If the problem is, in fact, a bleb-related infection, the first decision we have to make is whether the infection is isolated to the bleb and the anterior chamber, or involves the vitreous. If it involves the vitreous, then the problem is endophthalmitis and we switch to a completely dif-ferent treatment protocol. With endophthalmitis, the retina team usually takes over and the treatment typically involves intravitreal injections, topical antibiotics and possibly vitrectomy.
If the problem is isolated in the anterior chamber with no vitreous involvement—no cells behind the crystalline lens—then we start the patient on hourly, around-the-clock topical antibiotic treatment.
Antibiotic Protocol
In this situation, there are two primary options in terms of topical antibiotics. Some surgeons use fourth-generation fluoroquinolones such as moxifloxacin or gatifloxacin; others prescribe fortified topical antibiotics, meaning an agent such as vancomycin or cefazolin in combination with an aminoglycoside such as tobramycin or gentamycin. In most situations, I favor using fortified antibiotics.
In addition to the topical fortified drops, I generally also put the patient on an oral third-generation fluoroquinolone. The effectiveness of doing this is unproven, but when the stakes are this high I believe in using every tool that may help.
Most patients respond to this protocol—but not all. So, you need to monitor them daily. If you see a lot of inflammation and you’re very nervous, it’s worth checking the patient twice in the same day—morning and afternoon. An infection can evolve rapidly.
During this treatment period, you don’t want to attempt to address the leak, because the tissues are still infected; if you were to operate, there’s a chance you might make the situation worse. Your first goal is to sterilize the area; that takes precedence over sealing any leakage.
As an aside, the flow dynamics of a leak are another point of contention with regard to pathophysiology. Many leaks appear to only have continuous outflow (although this may depend on the size of the leak and the intraocular pressure), and very few bacteria can swim upstream. That’s another reason some surgeons suspect that the leak may have nothing to do with the infection, in terms of the inciting pathology. On the other hand, I’ve seen bleb leaks in which the fluid is visibly moving in and out; that might be a patient that you admit to the hospital and see twice a day.
Of course, it’s possible to culture the bacteria to try to identify it. I generally manage the patient more empirically, although culturing can be helpful in some situations. For example, if you’re using fortified antibiotics, depending on what species you find you’re dealing with, you might be able to stop one of the antibiotics. The reason for using two antibiotics in the fortified approach is that you want to treat with a drug that has good gram-positive coverage and another that has good gram-negative coverage. If your culture comes back gram-negative, then you don’t have to worry about gram-positive coverage and you can stop that antibiotic (or vice versa). However, a lot of physicians—probably more than half—use a single topical fluoroquinolone with broad-spectrum coverage. In that situation, it doesn’t matter what the bug is; you’re still going to keep up therapy and treat empirically.
Once treatment has been initiated, we see the patient every day for several days. If we see any evidence of vitreous involvement, we turn the patient over to the retina team.
Winding Down
If the vitreous doesn’t become involved and the patient is clinically improving, we slowly start tapering the frequency of the medication. Again, my choice of initial treatment is topical fortified antibiotics every hour around the clock. I’ll keep that up for a day or two until I’ve seen some recovery.
I don’t wait too long to begin tapering, because we’re asking the patient to get up every hour all night—or simply stay up all night—to take the drops. If you do that for more than one day, you start to have serious sleep-deprivation problems. That’s why you may want to admit some of these patients to the hospital. In the hospital the patient can try to sleep, and the nurse can come in every hour to administer the drops.
Once there’s been some recovery on a tapered dose, then I switch from the topical fortified to a fourth-generation fluoroquinolone at the same frequency. Even with the tapered dose, this regimen is still very labor-intensive; that’s why the clinician’s trigger to admit a patient to the hospital shouldn’t be too high. I realize that someone who practices out in the community might have to see the patient in a hospital which could be located at a distance and might not have an ophthalmologic exam lane, so this is an option that is subject to each surgeon’s circumstances and preference.
Another option is to refer the patient to a specialist or a surgeon who is positioned to more easily manage a hospitalized patient. Again, this is subject to a host of individual considerations. The bottom line is that if you have any concerns about admitting your patient to the hospital, it may be worth considering calling in a consult or referring the patient to a center that manages this type of situation more frequently.
To Revise or Not to Revise
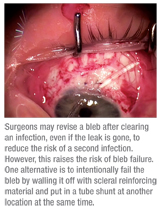
One thing we do know: Studies have found that once you’ve had an episode of blebitis, the risk of having a second episode is much higher.5 For that reason, most surgeons will revise the bleb once the infection has cleared, even if the leak is gone.
However, there’s a potential problem: Once you do a bleb revision, there’s a very significant chance that the bleb is going to fail. If you treat an infection and you still have a leak, that’s a good reason to strongly consider doing a bleb revision. But if you clear the infection and the leak is gone, then what should you do? Should you revise the bleb because the risk of getting another infection is so high? In general, the answer for me is yes—but not everyone agrees.
If you make the decision to intervene, you have two options. The first option is to simply do a bleb revision such as a conjunctival advancement. You open the area, excise the un-healthy bleb and then pull down new conjunctiva and close it. The second option is to intentionally fail the bleb by walling it off with some form of scleral reinforcing material and put in a tube shunt at another location at the same time.
When I make a decision about how to proceed in this situation, a key factor is the patient’s situation. If I think I’ll only have one chance to help the patient, then I’ll go for walling off the bleb and implanting the tube shunt. Likewise, if I think the risk of failure is high and the consequences of failure are high—for example, if the patient doesn’t have a lot of optic nerve left, so I really can’t afford periods of failure—then I’ll put in the shunt at the same time.
Making the Best of It
Despite the gaps in our understanding of the causes of blebitis, we have a good track record for pre-serving vision in those rare cases in which it does occur. Eventually, a simpler and safer replacement for trabeculectomy will become the new gold standard; until then, the vast majority of our patients should do very well.
Dr. Rhee is associate professor at Harvard Medical School and staff physician at the Massachusetts Eye & Ear Infirmary in Boston.
1. Jampel HD, Quigley HA, Kerrigan-Baumrind LA, Melia BM, Friedman D, Barron Y; Glaucoma Surgical Outcomes Study Group. Risk factors for late-onset infection following glaucoma filtration surgery. Arch Ophthalmol 2001 Jul;119:7:1001-8.
2. Georgoulas S, Dahlmann-Noor A, Brocchini S, Khaw PT. Modulation of wound healing during and after glaucoma surgery. Prog Brain Res 2008;173:237-54.
3. Jones E, Clarke J, Khaw PT. Recent advances in trabeculectomy technique. Curr Opin Ophthalmol 2005;16:2:107-13.
4. Wells AP, Cordeiro MF, Bunce C, Khaw PT. Cystic bleb formation and related complications in limbus- versus fornix-based conjunctival flaps in pediatric and young adult trabeculectomy with mitomycin C. Ophthalmology 2003;110:11:2192-7.
5. Ordan JL, Catey B, Melville MM, et al. Risk factors for development of post trabeculectomy endophthalmitis. Br J Ophthalmol 2000:84:1349-1353.



