New cross-linking protocols are attempting to circumvent the shortcomings of the Dresden protocol, in particular by aiming to decrease treatment time and reduce postop pain, while producing comparable results.
“I’m excited by the latest developments in epi-on cross-linking,” says Farhad Hafezi, MD, PhD, chief medical officer of the ELZA Institute, Dietikon, Switzerland. “Given everything we’ve learned about cross-linking over the years, we’re now approaching a point where we can leverage that knowledge and combine it with molecules that act as penetration enhancers (to get riboflavin across the epithelium and into the stroma in clinically effective levels), pulsed UV light (that lets oxygen diffuse back into the cornea) and smart, individualized cross-linking protocols to get corneal stiffening effects on the same level as the epi-off gold standard Dresden protocol. At my institute, we’ve already moved over to epi-on cross-linking.”
Neera Singal, MD, FRCS(C), an assistant professor, chief of cornea and the head of the collagen cross-linking program in the department of ophthalmology and vision sciences at the University of Toronto, is also eager for epi-on cross-linking to come to the fore. “Dr. Stulting and colleagues recently reported the results of an epi-on technique with a new riboflavin formulation and UV light, which seems promising but isn’t available yet for prime time,”1 she says. “With an efficacious epi-on technique, we’d virtually eliminate the risk of haze and have a significant improvement in postoperative pain. It’d also allow us to perform CXL on thinner corneas.”
Long-term studies are still needed to understand how all of these new protocols may affect corneal stability down the line,2 but epi-on, accelerated and pulsed protocols show promise. Here, we’ll look at some epi-on treatments in the FDA pipeline, and other cross-linking protocols in use outside the United States.
iLink
Glaukos’ epi-off iLink procedure is currently the only FDA-approved protocol for collagen cross-linking. The epithelium-off treatment involves a 30-minute riboflavin instillation (Photrexa Viscous, 0.146% riboflavin 5’-phosphate in 20% dextran ophthalmic solution) followed by a 30-minute, 3-mW/cm2 UVA irradiation.
As an epi-off procedure, iLink entails an epithelial defect. Because of this, patients often complain about slow healing and discomfort, says Brandon Ayres, MD, of Ophthalmic Partners in Bala Cynwyd, Pennsylvania. “Most patients feel discomfort for about three days, and during this time, they can’t go to work or do much of anything,” he says. “There’s also a risk of infection. However, if you don’t make some form of epithelial defect, you’re likely not doing the best for the patient because the CXL won’t be as successful.”
Glaukos’ next venture into cross-linking treatment is an epithelium-on procedure. The company announced positive Phase III trial results earlier this year. The epi-on procedure achieved its primary endpoint of demonstrating a Kmax treatment effect of -1 D at six months vs. placebo (p=0.0004). This included a 0.2-D improvement in Kmax in the treated arm and a 0.8-D worsening in Kmax in the placebo-controlled arm. Glaukos says this means that the epi-on treatment is effective at halting or slowing keratoconus disease progression. The company plans to submit an NDA in 2022.
Dr. Ayres, one of the doctors participating in the trial, says he’s excited about the approval of an epi-on cross-linking treatment. “Patients will experience less discomfort and faster recovery times,” he says. “Without having an epithelial defect, the risk of infection is much lower as well.”
The new epi-on version is different from iLink. This treatment involves two different proprietary riboflavin formulations. “We were masked to the two types of riboflavin in the study,” Dr. Ayres says. “Formulation A is likely a pretreatment meant for sensitizing the epithelium to allow for better penetration, and the addition of Formulation B may be meant to get further into the stroma.”
Dr. Ayres adds that the cross-linking is aided by a pair of special goggles that concentrate oxygen around the cornea. “Oxygen, riboflavin and UV light are required for cross-linking to work,” he explains. “If we can supply more oxygen, the thought is that we can more rapidly and effectively perform the CXL procedure. In the trial, we were able to do an entire CXL procedure in a little less than half the time we do it now, with significantly less discomfort and fewer healing issues because it’s not an epi-off procedure.”
The trial also used a new UV light device in a pulsatile fashion to give the oxygen levels in the cornea time to rise between light exposures. Pulsed light treatments aren’t approved in the U.S. yet, but many of these new protocols are making use of it to promote oxygen availability to the cornea. “The oxygen concentration must be at least 94 percent to cross-link,” Dr. Ayres says. “In the trial, we used a higher fluence of light, and therefore needed more oxygen. That’s where the hyperbaric goggles come in.
“I was impressed by the depth of the treatment we were able to get into the cornea,” he continues. “One of the major issues of epi-on cross-linking is that the riboflavin doesn’t penetrate as deeply, but we had good results in the trial. As a clinician, I felt like I was doing good. Deeper penetrance into the cornea means a better cross-linking treatment and a more stable cornea. We’re looking forward to seeing the compiled trial data soon, and hopefully in a few years, the FDA will green-light this new procedure. We really want epi-on treatments to be as effective or even more effective than epi-off treatments.”
Epismart
|
|
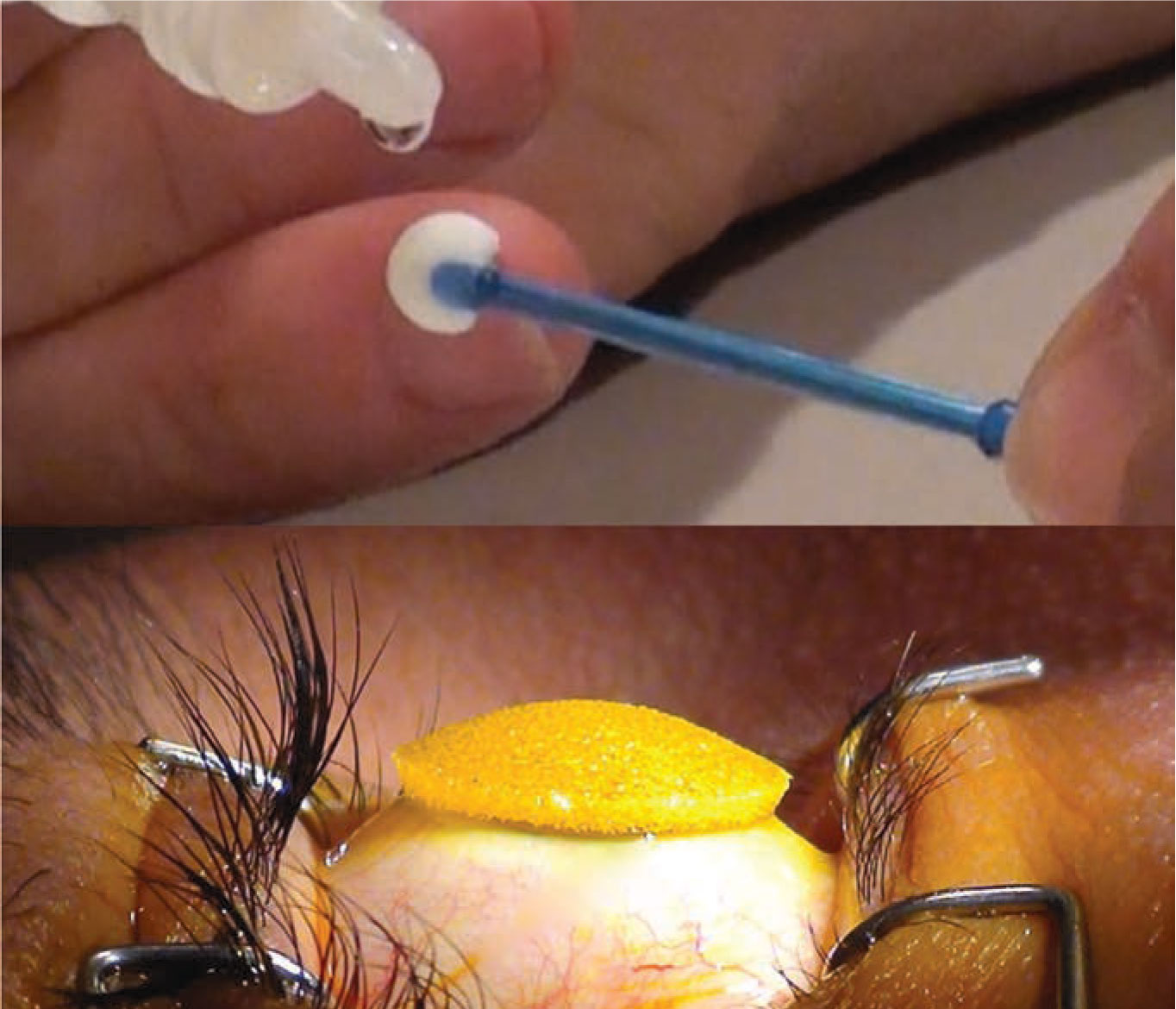
The Epismart system uses a disposable wand to remove the mucin layer and promote epithelial permeability (top). A loading sponge is then placed on the cornea to maintain optimal drug concentration (bottom). (Image courtesy of CXL Ophthalmics) |
EpiSmart (CXL Ophthalmics), an epi-on procedure developed by Roy S. Rubinfeld, MD, MA, is scheduled to begin Phase III trials in a few months. The treatment uses a proprietary riboflavin formulation that contains sodium iodide as its key active ingredient.3 “Sodium iodide enhances the penetration of riboflavin and helps to achieve homogeneous stromal loading,” explains Michael D. Webb, MA, MBA, president and CEO of EpiSmart. “It also scavenges hydrogen peroxide and converts it into oxygen. We apply the riboflavin solution, known as RiboStat, once at the initial outset of the procedure, rather than continually dropping riboflavin into the patient’s eyes.”
The EpiSmart procedure takes approximately 50 minutes from start to finish, with a 20-minute UVA exposure, and consists of three parts. First, a small wand-shaped tool is used to remove the mucin layer from the surface of the cornea. Next, the riboflavin solution is applied to a loading sponge that helps maintain drug concentration during stromal loading. Epismart uses a pulsing UVA device with two arms for bilateral treatment. It includes a fixation light and modulated compensating light to maintain the eye’s target focus.
“This is a true epi-on procedure,” Mr. Webb explains. “Generally, our patients go back to school or work the next afternoon with both eyes treated. We hope EpiSmart will help to move cross-linking toward a treatment paradigm that allows for treatment upon initial diagnosis, rather than waiting for significant vision loss.”
On-Eye Cross-linking
“An operating room in a contact lens” is how Roy S. Chuck, MD, PhD, the Paul Henkind Chair and a professor in the department of ophthalmology and visual sciences at Montefiore-Einstein, and chairman and cofounder of TECLens, describes the CXLens. The scleral lens-based device is connected via a thin fiber optic cable to a small, portable UV delivery device. No speculum is involved. The developers say that the familiar contact lens form helps to reduce patients’ fear of the procedure.
CXLens is meant to be a comfortable procedure. “Patients can move their head, eyes, and even close the treated eye during therapy, all without interrupting or displacing the UV beam,” says Dr. Chuck. “The scleral lens tracks eye movement, so when the eye moves, the device moves. This increases the accuracy of UV targeting. With conventional cross-linking, if the eye moves out of the field of the light, you get no effect.”
|
|
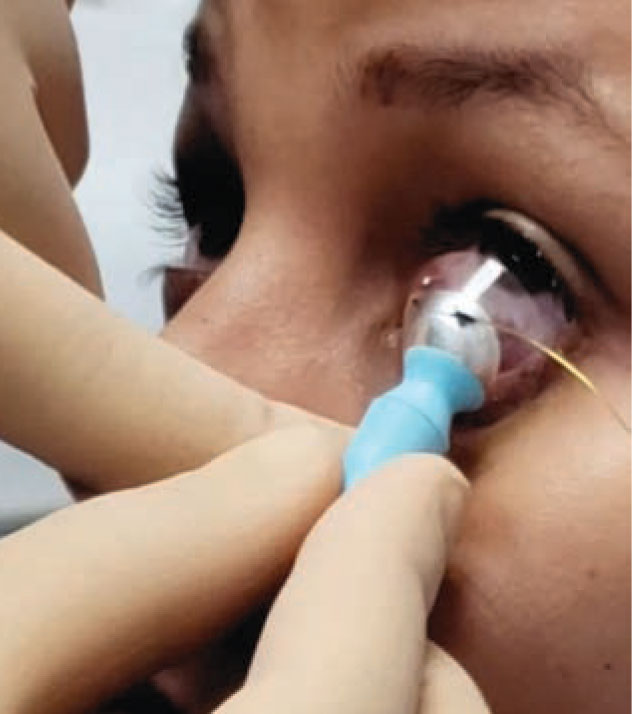
The CXLens is a scleral lens-based device that emits UV light to cross-link the cornea. This image shows lens insertion. (Image courtesy of TECLens) |
Before the procedure, a reservoir contact lens filled with riboflavin is placed on the eye to hold the riboflavin against the cornea for absorption. The riboflavin doesn’t have to be readministered during the treatment, nor does the treatment require additional oxygen. “We preload the cornea with perfluorocarbon, which can carry three times as much oxygen as is in the air,” explains Patrick D. Lopath, MBA, MS, a biomedical engineer, COO and co-founder of TECLens.
A small pilot study of the CXLens with Juan Batlle Logroño, MD, in the Dominican Republic demonstrated successful results, says Mr. Lopath. Nine corneal transplant candidates with advanced keratoconus received a scleral contact lens reservoir containing 0.007% benzalkonium chloride preserved with 0.25% riboflavin-monophosphate for 30 minutes. The reservoir lens was then replaced with the CXLens UVA light-emitting contact lens, and the eye was irradiated with 375-nm UVA light at 4 mW/cm2 intensity for 30 minutes for a dose of 7.2 J/cm2.4
After six months, the treated eyes had an average of -1 ±1.6 D decrease in maximum keratometry (p=0.049), a nonsignificant 2.3 ±7.5-letter improvement in BCDVA (p=0.19), a nonsignificant -17 ±14-µm decrease in thinnest corneal thickness (p<0.01), and a nonsignificant -86 ±266-cells/mm2 decrease in endothelial cell density (p=0.2). The authors concluded that the device has the potential to perform efficient, high-throughput transepithelial corneal cross-linking and is ready for larger-scale studies. (You can view a video of the procedure in the online version of this article at reviewofophthalmology.com.)
Biomechanics in Real Time
|
|
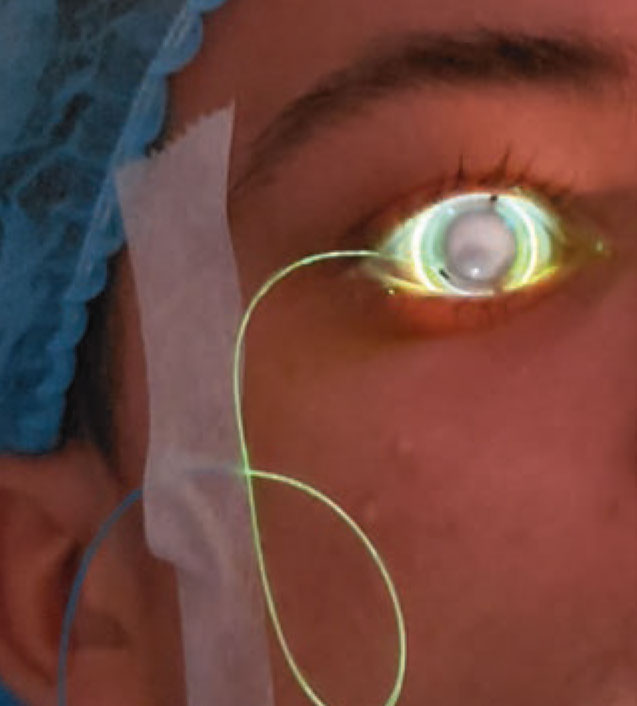
A fiber optic cable connects the CXLens to the UV delivery device. Patients can blink, close their eyes and turn their heads during the treatment because of the on-eye configuration and the device’s built-in eye tracker. (Image courtesy of TECLens) |
In addition to patient comfort and eye tracking, the CXLens’ on-eye treatment enables real-time sensing of the cross-linking-induced biomechanical changes without interrupting treatment. “We can measure this with our real-time biomechanical modeling feedback system,” says Mr. Lopath. “We measure how the ultrasound interacts with the eye and can deduce from that how much we’ve increased the tensile modulus of the corneal tissue.”
They say that CXLens also has the potential to be a noninvasive vision-correction technology, because it can alter corneal biomechanics and thus change the eye’s refractive shape. “We refer to the procedure as ‘quantitative cross-linking,’ ” says Mr. Lopath. “We use computational modeling to optimize a refractive treatment, since we know the changes that cross-linking makes to the eye.”
Reshaping while strengthening the cornea with UV light is a hot topic, says Dr. Ayres. “The UV lights we have in the States have 8-mm beams, which are too broad for targeted reshaping, but with a more focused beam of light applied to only certain parts of the cornea, we could predictably reshape it.”
To perform a refractive procedure using the CXLens, Mr. Lopath says the patient’s corneal topography is mapped with any standard topographer; then, that data is mapped onto a digital biomechanical framework built with data from healthy eyes with similar refractive error. “TECLens’ initial approach will use this population-based model because the equipment needed for measuring corneal biomechanics isn’t ubiquitous in ophthalmology practices today,” says Mr. Lopath. “For example, presbyopic treatment plans use a dataset of baseline biomechanics from presbyopic eyes.
“A computational model then iterates through different cross-linking treatments ‘in silico’ until the parameters for the best refractive outcome for a specific patient are determined,” he continues. “This treatment plan consists of a simple set of parameters such as UV pattern, intensity and time, allowing the physician to simply apply the appropriate CXLens and engage the control console to execute the plan. The control system uses the ultrasound feedback to stop the therapy when the baseline corneal stiffness has changed by the amount the digital model calculated for the optimum outcome.”
“As a refractive surgeon, I find that lower myopes and presbyopes are always the most hesitant to undergo ablative vision correction,” says Dr. Chuck. “We hope that this non-ablative, familiar contact lens-based procedure will open up a lot of eyes, if you will.”
Treating the Underlying Cause
Bala Ambati, MD, PhD, MBA, of Pacific Clear Vision Institute in Eugene, Oregon, a professor and the director of ophthalmology and visual science at the University of Oregon’s Knight Campus, and iVeena Delivery Systems’ president, began looking into the underlying causes of keratoconus when he felt unsatisfied by conventional cross-linking.
“In 2015, we did an experiment in my lab, then at the University of Utah, and found that we could use copper to increase lysyl oxidase,” he says. “In keratoconic patients, there’s a deficiency of lysyl oxidase, which leads to a deficiency of natural collagen cross-links. I felt that treating the underlying pathophysiology of the disease by enhancing the activity of this critical enzyme would be a great pharmacologic approach.
“Lysyl oxidase is a cuproenzyme,” he explains. “It’s dependent on copper ions for its function, and interestingly enough, keratoconic corneas have been described as being deficient in copper. There’s a variety of reasons for that, especially in places like the Middle East, where there’s a lot of exposure to alkaline sand, which can affect copper transport throughout the cornea.”
He went on to develop IVMED-80, currently an orphan drug candidate for keratoconus. “A pharmacologic approach can avoid surgery and the risks and pain associated with it,” he says. IVMED-80 is a prescription aqueous soluble drop. In clinical trials, there were no reports of irritation or effects on vision. Patients can instill the drops themselves, just like glaucoma drops.
Treatment duration will likely depend on disease severity and age at onset. “We know from surgical CXL studies that teenagers and adults who undergo surgical cross-linking have a fairly significant rate of relapse and need retreatment with repeat surgical CXL within five years. I suspect IVMED-80 will be patient-dependent, where patients who present with KCN in their 30s may only need it for a year. Patients who present when they’re teenagers might need it for a very long time.”
The Phase I/IIa study was conducted in Mexico and included 31 patients who had completed a course of IVMED-80, administered twice daily. Three subarms received placebo (artificial tears), IVMED-80 for six weeks or IVMED-80 for 16 weeks. All patients were followed for 26 weeks to determine the impact of duration of therapy, as well as the effects of therapy cessation.
“As expected, the placebo group progressed by about 0.2 D over the course of the six months,” says Dr. Ambati. “The patients who received IVMED-80 for six weeks had no benefit; they pretty much wound up where they started, in terms of Kmax. The patients who received IVMED-80 for 16 weeks improved. Their corneas flattened by about 0.8 D over the follow-up period. We’re very pleased with these results. We had 1 D of reduction relative to placebo over the follow-up period and there were no adverse events. In addition to Kmax flattening, we observed a reduction in corneal astigmatism, as measured by Pentacam, by about 0.5 D as well. That was an unexpected benefit.”
At the end of the six months of follow-up, the IVMED-80 group receiving drops for 16 weeks had an 11.3-letter improvement, relative to baseline. The placebo patients had an eight-letter improvement relative to baseline. “The reason both groups improved is because patients memorize the ETDRS eye charts,” says Dr. Ambati. “But the treated patients improved more than the placebo patients, and the amount of improvement we saw with IVMED-80 was slightly higher than what was published in the U.S. clinical trial with Photrexa, the surgical CXL product, so we were encouraged by that and by the anatomical improvement as well.”
Dr. Ambati says he hopes patients with mild, moderate and even advanced keratoconus will benefit from this drop. “If the keratoconus is very severe or the patient has corneal scarring, they’ll need a transplant, of course, but as long as that’s not the case, and we can provide pharmacologic benefit at minimal risk, I think that would be a tremendous advance in our care of keratoconus patients.”
An at-home prescription drop could benefit clinicians as well. “Surgical cross-linking is a challenge to clinician workflow, to put it charitably, because of how long it takes to load riboflavin,” says Dr. Ambati. “It’s a challenge in terms of the number of postop visits, and it’s also a challenge to get insurance approval. If we can conserve physician time and energy and move away from a surgical paradigm to a pharmacologic paradigm, I think it’s a win for physicians, who would prefer not to have surgical risks and complications in patients that need to be managed, and who frankly have better uses for their time than putting in eye drops every two minutes for 45 minutes to load a patient’s cornea.”
CXL at the Slit Lamp
Though it’s a novel approach outside the United States, cross-linking at the slit lamp doesn’t differ practically from cross-linking in the OR. What makes cross-linking possible at the slit lamp is the photochemical reaction between UV light and riboflavin, which kills pathogens,5 explains Dr. Hafezi. “The patient’s position doesn’t matter when it comes to UV irradiation, and riboflavin doesn’t redistribute in the cornea for at least an hour when the patient is sitting upright,6 which is long enough to not only perform Dresden protocol cross-linking (30 mins UV irradiation) but greatly in excess of what’s required to perform accelerated cross-linking protocols, which are on the order of 10 minutes, not 30. Patients are comfortable sitting in a chair for this time, and they also have the advantage of being able to fixate their fellow eye on the slit lamp’s red fixation light.”
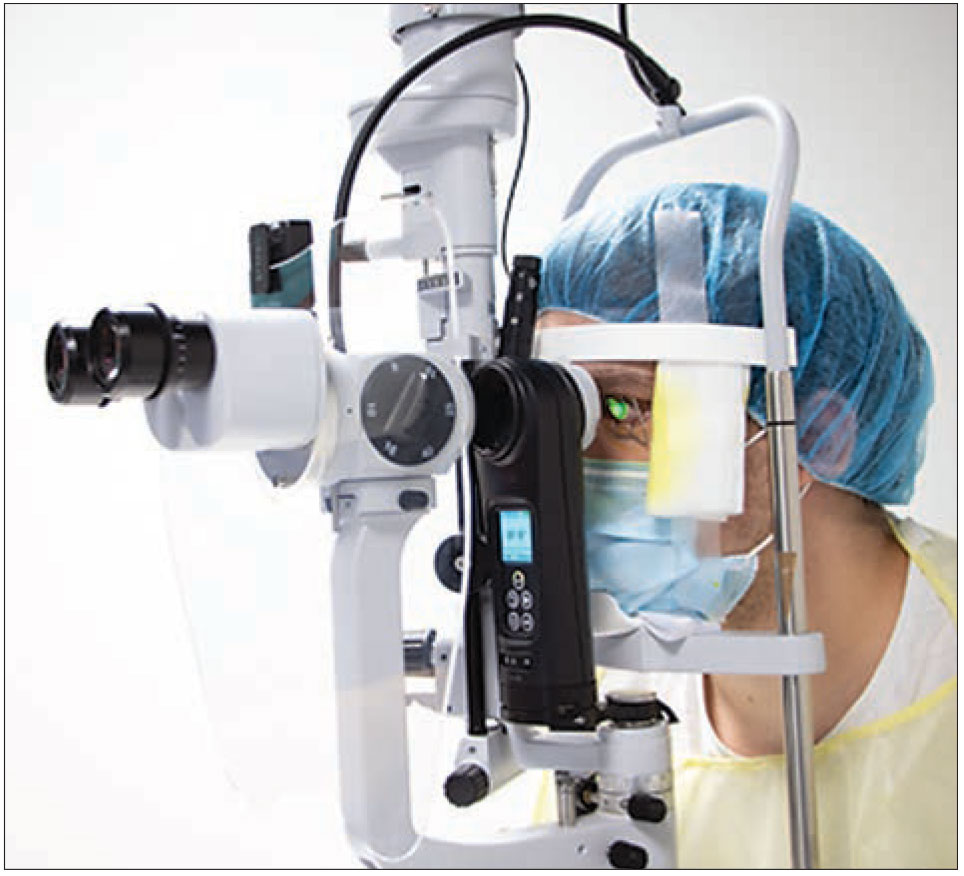
|
|
Performing cross-linking at the slit lamp is possible, in part, due to the photochemical reaction between UV light and riboflavin, which kills pathogens. Because it can be done outside the OR, experts say this approach will help to bring cross-linking to regions of the world that lack large hospital centers. (Image courtesy of Farhad Hafezi, MD, PhD) |
The main advantages of cross-linking at the slit lamp are cost and accessibility. Performing the procedure in-office removes the costs of running the OR, as well as the administrative burden of booking in competition with other surgeons, explains Dr. Hafezi. “It’s been hard to ignore the cost and efficiency benefits that have been seen with in-office cataract surgery and the move from performing intravitreal anti-VEGF injections from the OR into a procedure room, and we haven’t seen any additional safety issues associated with those transitions,” he points out. “It enables surgeons to be more flexible regarding when they can perform cross-linking, since they don’t have to wait for an OR slot to become available.”
Using a slit lamp will also open up cross-linking to more remote regions. Dr. Hafezi says that, given that most ORs are in big hospitals in large population centers, if you don’t need an OR, and just need a slit lamp, then you can perform cross-linking just as easily in remote parts of low-to-middle income countries as you can in a large hospital in a high-income country.
Currently, only one cross-linking device can be used at the slit lamp—the C-eye (EMAGine AG, Zug, Switzerland). C-eye is a portable, battery-powered device that can be charged with a USB-C cable. It automatically calibrates UV output and fits a range of slit lamps. The device includes eight keratoconus protocols, including the Dresden protocol, pulsed and high fluence protocols, and the sub400 thin-cornea protocol;7 a refractive cross-linking protocol for LASIK, SMILE and PRK; and two infectious keratitis treatment protocols.
“My go-to protocol for many years was 9 mW/cm2 for 10 minutes,” Dr. Hafezi says. “It doesn’t provide the same biomechanical strength as the Dresden protocol, but clinically, it’s sufficient for most forms of keratoconus in adults. I reserve the classic 30-minute Dresden protocol for the most aggressive forms of keratoconus (in children), but this will change in the near future. Our latest research, which is in press with TVST, identified an accelerated protocol using very high fluence that rivals the stability of the Dresden protocol but takes a fraction of the time. This protocol will be implemented clinically in the next one to two years. In patients with corneas thinner than 400 µm, I regularly perform our sub400 protocol epi-off CXL.”
Branching into Thin Corneas
Other Approaches To and Uses For Cross-linking Here are two more off-label ways cross-linking is being used: • Oral riboflavin. John S. Jarstad, MD, FAAO, of the University of South Florida-Tampa, proposed a walking protocol for cross-linking that involves taking 400 mg/day of riboflavin and walking vigorously toward the sun for 15 minutes each day without wearing sunglasses or UV-blocking contact lenses. In his clinical trial, all patients following this protocol achieved an average 1.2 D of corneal flattening and their keratoconus didn’t progress, while the control group taking 100 mg/day stabilized but saw less flattening. (To read more about this, see “Crosslinking 2020: Closer to the Holy Grail” from the October 2020 issue of Review.) • PACK-CXL. As Dr. Hafezi points out, the photochemical reaction between UVA light and riboflavin during cross-linking has the beneficial side effect of killing pathogens, making cross-linking a safe procedure to perform in an office setting. Photo-activated chromophore for infectious keratitis, or PACK-CXL, has been studied for the management of infectious keratitis and may also serve as an adjunctive therapy for treating fungal keratitis.14 A 2013 meta-analysis reported that cross-linking’s effectiveness in reducing corneal melt was in the following order, from most to least effective: Gram-negative bacteria, Gram-positive bacteria, Acanthamoeba and fungus.15 Researchers believe that its low effectiveness in fungal infections may be due to the fact that fungal infections penetrate deeper into the cornea than most bacteria. One case study in 2019 demonstrated resolution of a fungal infiltrate at the site of a phaco-tunnel.16 The cross-linking procedure used 0.1% riboflavin with the Dresden protocol. Rashmi Deshmukh, MD, of Queens Medical Centre, University of Nottingham, says it’s possible that PACK-CXL had a synergistic effect with the already administered antifungal medical treatment. —CL |
Conventional cross-linking is limited to eyes with a corneal thickness greater than 400 µm, but there are several techniques in use for thin corneas such as using hypo-osmolaric riboflavin to swell the cornea,8 a riboflavin-soaked contact lens9 and leaving epithelial cells in “islands” above the thinnest points.10
In 2014, Dr. Hafezi and his group began looking into a different approach, one that would alter the amount of light delivered to the eye, rather than altering the thickness of the cornea, which may be unpredictable. “When CXL was introduced, we didn’t have a complete understanding of the UV-riboflavin reaction,” he says. “We didn’t know, until our research group showed five years ago, that oxygen diffusion into the cornea was an essential component of the cross-linking reaction and that it gets consumed rapidly once the UV irradiation begins.11
“Once we understood that, this enabled us to develop a cross-linking algorithm that accounted for corneal thickness, UV intensity and duration of irradiation, and predicted the depth of the cross-linking effect.12 We then validated this algorithm, later called sub400, first in laboratory experiments and then in a clinical trial.7 This means that once we measure a cornea’s thickness, we can individualize the amount of UV irradiation to the cornea to cross-link a ‘safe’ amount of cornea, and leave a 70-µm safety margin of un-cross-linked cornea above the corneal endothelium. All of this can be performed at the slit lamp. I perform sub400 cross-linking at the slit lamp almost every time I spend a day performing surgery.”
Accelerated CXL
Proponents say that accelerated CXL addresses many of the problems found with conventional CXL such as the standard treatment’s long procedure duration, stromal damage, patient discomfort and risk of corneal haze.
“Accelerated cross-linking has really changed the way the procedure is offered to patients with keratoconus,” says Dr. Singal. “Most of the modifications that have been applied since cross-linking’s inception in 2003 are related to modifications in fluence and time, per the Bunsen-Roscoe law of reciprocity, so the total irradiation remains constant. The gold standard of CXL is the Dresden protocol, which is based on supplying the cornea with a total irradiation of 5.4 J/cm2. Accelerated CXL can decrease the procedure time by increasing the fluence, while keeping the total irradiation amount constant at 5.4 J/cm2.”
Dr. Singal says that because the treatment duration is much shorter, the procedure is more efficient and comfortable for the patient. “The shorter time also reduces corneal dehydration, and less keratocyte exposure time potentially results in less haze,” she adds.
However, simply following the Bunsen-Roscoe law of reciprocity for accelerated CXL protocols doesn’t lead to the same outcomes as in the standard Dresden protocol. “This is because we’re shortening the time that oxygen is available to the cornea during the CXL process,” Dr. Singal says. “Recent studies have shown that pulsed delivery of the UVA light to the cornea is important for accelerated CXL because it allows for more oxygenation during treatment. This results in enhanced release of singlet oxygen, allowing for more effective cross-linking of the collagen molecules.”
Dr. Singal’s 2020 study on accelerated epi-off cross-linking prospectively studied efficacy, risk of progression and characteristics affecting outcomes in 612 eyes.13 “Our study is the largest prospective study looking at accelerated CXL in keratoconus patients,” she notes. “It was reassuring to see that our study supported many of the smaller studies, regarding efficacy. We found that our cohort showed stabilization of the disease at one year, which we defined as a change of less than
1 D in Kmax from baseline.”
When determining progression risk in the study, she says age and preop Kmax values were important. “The risk of progression for our entire cohort was 17.9 percent, which is consistent with previously reported studies using the accelerated protocol,” she says. “However, when we looked at our subset of patients that had a preop Kmax value greater than 58 (191 eyes, approximately 30 percent of the total group), we found that about 20 percent progressed at one year. Among our subset of pediatric patients (14 to 18 years old, 53 eyes), we noticed a very high risk of progression at 32 percent.” Dr. Singal says it’s important to counsel these patients preoperatively about the increased risks and closely monitor them after the procedure.
Dr. Ayres is a consultant for Glaukos. Dr. Hafezi is a shareholder of EMAGine AG and holds patents for a corneal CXL apparatus and a chromophore for CXL application. Dr. Singal has no relevant financial disclosures.
1. Stulting RD, Trattler WB, Woolfson JM, Rubinfeld RS. Corneal cross-linking without epithelial removal. J Cataract Refract Surg 2018;44:11:1363-1370.
2. Pasha H, Palazzolo L, Prakash G. Update on corneal collagen crosslinking for ectasia. Curr Opin Ophthalmol 2021;32:4:343-47.
3. Rubinfeld RS, Stulting RD, Gum GG, Talamo JH. Quantitative analysis of corneal stromal riboflavin concentration without epithelial removal. J Cataract Refract Surg 2018;44:2:237-242.
4. Dackowski EK, Batlle Logroño J, Rivera C, et al. Transepithelial corneal crosslinking using a novel ultraviolet light-emitting contact lens device: A pilot study. Transl Vis Sci Technol Special Issue 2021;10:5:5:1-10.
5. Wollensak G, Spoerl E, Reber F, Seiler T: Keratocyte cytotoxicity of riboflavin/UVA-treatment in vitro. Eye (Lond) 2004;18;7:718-722.
6. Salmon B, Richoz O, Tabibian D, Kling S, Wuarin R, Hafezi F: CXL at the Slit Lamp: No clinically relevant changes in corneal riboflavin distribution during upright UV irradiation. J Refract Surg 2017, 33;4:281.
7. Hafezi F, Kling S, Gilardoni F, Hafezi N, Hillen M, Abrishamchi R, Gomes JAP, Mazzotta C, Randleman JB, Torres-Netto EA. Individualized corneal cross-linking with riboflavin and UV-A in ultrathin corneas: The sub400 protocol. Am J Ophthalmol 2021;224:133-142.
8. Hafezi F, Mrochen M, Iseli HP, Seiler T: Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas. J Cataract Refract Surg 2009;35;4:621-624.
9. Jacob S, Kumar DA, Agarwal A, Basu S, Sinha P, Agarwal A: Contact lens-assisted collagen cross-linking (CACXL): A new technique for cross-linking thin corneas. J Refract Surg 2014;30:6:366-372.
10. Mazzotta C, Ramovecchi V: Customized epithelial debridement for thin ectatic corneas undergoing corneal cross-linking: epithelial island cross-linking technique. Clin Ophthalmol 2014;8:1337-1343.
11. Richoz O, Hammer A, Tabibian D, Gatzioufas Z, Hafezi F: The biomechanical effect of corneal collagen cross-linking (CXL) with riboflavin and UV-A is oxygen dependent. Transl Vis Sci Technol 2013;2:7:6.
12. Kling S, Hafezi F: An algorithm to predict the biomechanical stiffening effect in corneal cross-linking. J Refract Surg 2017;33:2:128-136.
13. Hatch W, El-Defrawy S, Tone SO, et al. Accelerated corneal cross-linking: Efficacy, risk of progression, and characteristics affecting outcomes. A large, single-center prospective study. Am J Ophthalmol 2020;213:76-87.
14. Deshmukh R. Commentary: PACK-CXL in fungal keratitis. Indian J Ophthalmol 2019;67:10:1701-02.
15. Alio JL, Abbouda A, Valle DD, Del Castillo JM, Fernandez JA. Corneal cross linking and infectious keratitis: A systematic review with a meta-analysis of reported cases. J Ophthalmic Inflamm Infect 2013;3:1:47.
16. Thakur A, Gupta A, Handa S. Application of photoactivated chromophore for infectious keratitis-corneal collagen crosslinking for fungal phaco-tunnel infection. Indian J Ophthalmol 2019;67:10:1700.