Here’s a common scenario in many ophthalmologists’ offices: A patient is referred for evaluation of narrow angles. The patient is middleaged and totally asymptomatic; her vision and pressures are fine. Her visual fields and optic nerve OCTs all look OK. You look at the back of her eyes without dilating her pupils and the optic nerves appear totally healthy. Gonioscopy confirms the presence of narrow angles, but there are no peripheral anterior synechiae. She doesn’t have glaucoma. The only thing the patient has is appositional closure—a narrow angle.
Ninety-nine out of 100 times, this person will receive a laser peripheral iridotomy. The reason for this is that an LPI is generally seen as a proactive way to minimize the likelihood of a future acute angle-closure attack. (Eliminating pupillary block may also delay the progression from primary angle closure suspect to primary angle closure or glaucoma.) The problem is, an LPI isn’t consequence-free, and there’s little direct evidence-based data to support doing LPI in this situation.
Here, I’d like to review the existing data and discuss the reasoning behind this choice of action, in hopes of encouraging you to think twice before automatically performing an LPI, simply because a healthy patient has a narrow angle.
The Rationale for Treatment
As doctors, we often prefer to err on the side of caution with our patients. The practical steps we take in a given scenario, of course, are usually based on the accepted wisdom about that situation. For example, when I was a resident 20 years ago, we believed that any intraocular pressures over 21 mmHg needed to be brought down. So, every patient that came into the clinic with a pressure of 22 or 23 mmHg would get a prescription for Xalatan, even if those individuals had a normal visual field and their optic nerves looked fine.
The truth was, we really had no proof that this was beneficial until the Ocular Hypertension Treatment Study came out. OHTS was a large, multicenter, randomized, prospective trial designed to reveal whether medical treatment lowered the risk of glaucoma development. It turned out that it does—but it only cuts the risk of glaucoma from 9.5 to 4.4 percent. The take-home conclusion of OHTS was that most patients who have elevated IOP and nothing else don’t need to be treated (although treatment does make sense for a subset of patients with high-risk characteristics such as thin corneas). Because of that study, today we typically just follow most of those patients instead of treating them.
Unfortunately, when it comes to patients with narrow angles, we don’t have a study equivalent to OHTS. We don’t actually know how many future angle-closure attacks we’re preventing by performing LPIs. That’s why we can’t say to a patient with narrow angles, “Mrs. Smith, your risk of going blind is X percent (or your risk of getting glaucoma is Y percent), but the odds will improve by this much if I perform this procedure.” We don’t have the numbers to support that, so we just treat everybody.
The fact that we can now create an iridotomy with a laser is a big part of the reason for the current approach. Back in the days before lasers an iridectomy required taking the patient to the OR, opening the eye and snipping a piece of iris. That’s obviously pretty invasive and risky. Since the risk/benefit ratio wasn’t ideal, we used provocative testing to try to figure out which patients were likely to go into acute angle closure. We’d make people drink a gallon of water or sit them in a dark room, or more commonly, perform pharmacologic testing. These tests were helpful for identifying patients at the highest risk of angle-closure attacks, but they weren’t perfect. (Based on these tests, gonioscopic criteria for high-risk patients were developed. Today, anterior chamber OCT can also be used to identify severely narrowed angles, which are generally presumed to be at high risk for an attack.)
Then the laser came along. Now we could create a hole in the iris without having to enter the eye, and the risk/benefit ratio was suddenly very different. So we stopped doing provocative tests. Now we just take a narrow-angle patient over to the YAG laser and do a quick treatment. The relative safety and ease of laser iridotomy has made it seem like a no-brainer. However, just as we overtreated ocular hypertension in the past, we’re probably doing too many laser iridotomies in these patients.
 |
The Levels of Angle Closure
Patients with narrow angles fall somewhere along a continuum, based on a simple staging system. There are three distinct stages in the angle closure disease process: primary angle closure suspect; primary angle closure; and primary angle closure glaucoma. An individual’s place on the continuum is determined by several factors, including how much appositional contact there is between the iris and the trabecular meshwork; whether the angle has peripheral anterior synechiae or PAS; whether the IOP is elevated; and the condition of the optic nerve. (See table, above.)
• If an individual has appositional contact but no PAS, and a normal IOP and optic nerve, he’s considered a primary angle closure suspect or PACS. This is the most common scenario we’re likely to encounter in the clinic.
• If someone has a high IOP and/ or PAS (i.e., trabecular meshwork dysfunction) but no optic nerve damage, that’s considered primary angle closure.
• If someone has all of the above and optic nerve damage, that’s considered angle-closure glaucoma.
As it turns out, we have good clinical evidence to guide us when managing primary angle closure and angle-closure glaucoma, but not when managing PACS. The treatments at each stage are very different. We know that cataract surgery is beneficial for angle-closure glaucoma, so we treat these patients with cataract surgery combined with filtering surgery— either a trabeculectomy or tube, if the amount of optic nerve damage is severe and the patient requires a low target IOP. (MIGS, a.k.a. minimally invasive glaucoma surgery, may have a role to play in addressing milder stages of PACG, but we don’t know for sure; that’s still under investigation.) For primary angle closure, the second stage, we have a large, prospective, international multicenter trial called the EAGLE study. (EAGLE stands for Effectiveness in Angle closure Glaucoma of Lens Extraction.)1
In the EAGLE trial, primary angleclosure patients age 50 and older, with pressures above 30 mmHg but no cataracts, were randomized to clear lens extraction or LPI (the latter being the standard of care). Surprisingly, the patients that underwent clear lens extraction, even when they were 20/20, did better than patients who received laser. They had lower pressures, more open angles, and they didn’t need as many medications as the laser group. In the phaco group, 21 percent needed additional treatment; in the LPI group, 62 percent needed additional treatment. In addition, the cost-effectiveness of treatment and the patients’ quality of life were slightly better in the phaco group.
Based on the EAGLE results, if I have a patient with primary angle closure, I’ll recommend cataract surgery if the pressure is high, even if the patient doesn’t have a cataract. (My criteria for “high pressure” in this scenario is above 30 mmHg, because that was the inclusion criteria in the study. It’s not clear whether we could extrapolate that to a pressure lower than 30 mmHg.)
The bottom line is that we have good clinical evidence to guide our treatment decisions in those two scenarios. Unfortunately, we don’t have any good evidence for how to manage a PACS patient. There is no “Primary Angle-Closure Suspect Treatment Study,” which means there’s no clinical evidence that any surgical treatment for patients with PACS—including LPIs—is beneficial.
Some relevant data does exist, but it’s difficult to apply it directly to the situations we encounter in the clinic. For example, one study called the Zhongshan Angle Closure Prevention Trial (ZAP for short) was conducted in China.2 ZAP was a welldesigned, prospective, randomized trial involving 775 PACS patients. The researchers performed LPI in one eye whle leaving the other eye alone to serve as a control. As expected, the laser treatment opened the angle, but even after laser treatment the angles continued to narrow at the 18-month follow-up period in the study. Interestingly, out of 775 patients, none of the individuals at risk suffered acute angle closure. That’s important, because if you want to prevent something bad from happening, you first need to know the risk that the event will occur.
Longer follow-up data from the ZAP study has been submitted for publication, but right now we don’t know how many untreated PACS patients developed acute angle closure, elevated IOP or synechial formation. ZAP is the first prospective, randomized trial to examine whether LPI in PACS is beneficial, but it was conducted in Chinese patients and may not be generalizable to an American population.
Another study conducted in India estimated that about one in five patients progresses from PACS to primary angle closure at five years, and about one in four of those patients goes on to develop glaucoma.3 Again, these numbers may not apply to an American population.
The Pros and Cons of an LPI
Let’s consider the pros and cons of performing an LPI in PACS patients. Arguments in favor of performing an LPI include:
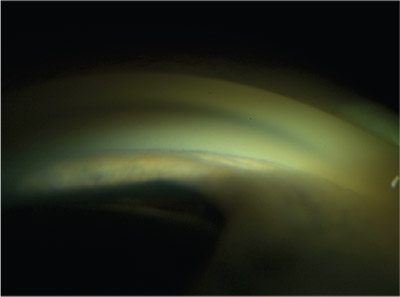 |
| Gonioscopic view of a narrow angle. None of the anatomical angle landmarks, such as the trabecular meshwork or scleral spur, are visible. There is a significant amount of forward convexity of the iris. Compression gonioscopy would distinguish between appositional (reversible) and synechial (irreversible) closure. |
• Acute angle closure is a potentially blinding ocular emergency. It’s one of the few emergencies in ophthalmology. Pupillary block is thought to be a major component of such an attack, and doing an iridotomy does eliminate pupillary block. Furthermore, angle-closure glaucoma is an aggressive disease, and probably the leading cause of glaucoma blindness in the world. Open-angle glaucoma is much more common than angle-closure glaucoma in America, but there are so many angle-closure patients in China and India that overall, more people on the planet go blind from angle-closure glaucoma than open-angle glaucoma. So at least in those countries, it’s a very relevant problem. By doing an LPI, you’re treating a preventable cause of glaucoma blindness.
• LPI in PACS probably prevents progression to PAC. As mentioned previously, PACS, PAC and PACG represent sequential stages along a continuum of angle-closure disease. This disease process is progressive and driven by pupillary block, phacomorphic factors and other mechanisms. Eliminating pupillary block should delay or prevent the conversion of PACS to PAC.
• An LPI is fairly benign. Performing a laser peripheral iridotomy usually only takes a couple of minutes in the office or clinic, and the risks of infection or bleeding are minimal.
On the other hand, arguments against performing an LPI in PACS patients include:
• An LPI isn’t totally benign. The patient can develop iritis or an IOP spike. (In fairness, both of those things are treatable.)
Probably the worst thing that can happen, in my experience, is that in rare cases patients get extra spots of light in their vision—dysphotopsias. These are permanent, and they can be highly annoying to the patient. There’s really no way to fix them, except to surgically close the iridotomy or take out the crystalline lens.
Although this complication may sound minor, I’ve actually had patients leave my practice because the LPI I performed caused this to happen. (Of course, switching doctors doesn’t necessarily help: One patient left my practice and saw another glaucoma specialist in my area. The second doctor did the laser in the other eye and the same thing happened!)
• An LPI can accelerate cataract development. Doing an iridotomy will increase the rate of cataract progression because you’re altering the flow of aqueous in the eye.4,5 Aqueous bathes the lens in nutrients and when you alter its path, you’re going to accelerate cataract development. Of course, here in America this isn’t necessarily a major issue because most people have access to high-quality cataract surgery. On the other hand, if you’re talking about doing iridotomy on tens of thousands of Chinese or Indian people, you’re going to create a lot of cataracts in a relatively resourcepoor environment. Those individuals may not easily be able to get cataract surgery.
•AnLPImayleadtothedevelopment of posterior synechiae. An iridotomy allows aqueous to flow from the posterior to anterior chamber without passing through the pupil. This may lead to greater contact between the iris and lens near the pupil margin, thereby predisposing the eye to the formation of posterior synechiae, which could make future cataract surgery more difficult.
• An LPI only treats pupillary block, which is just one mechanism of angle closure. There are multiple mechanisms involved in angle closure. Pupillary block is probably the most common mechanism in Western nations, but in the Chinese and Indian populations there may be other contributing factors such as the size or vault of the lens, or the position or insertion of the ciliary body. Iridotomy does not address these components, which may explain the continued angle narrowing of treated eyes in the ZAP study. LPI is only treating part of the problem, and by accelerating cataract formation, it may be increasing the phacomorphic component.
What’s the Risk of an Attack?
Obviously, a key part of justifying an LPI in an asymptomatic patient is the idea that we’re lowering the risk of a potential future acute angle closure attack. But the value of our intervention depends in large part on how serious that risk is.
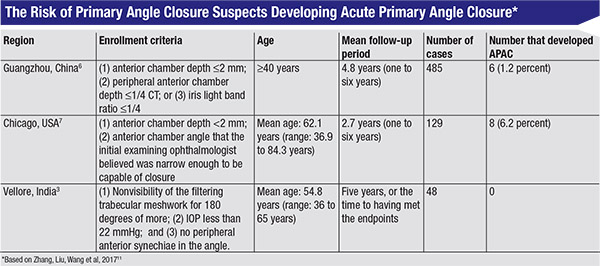 |
Three longitudinal studies from around the world may help to shed some light on this question. (See table, above.) One study conducted in Guangzhou, China, found that six out of 485 PACS patients—or 1.2 percent—later had an acute angle-closure attack.6 A study done in Chicago found that eight out of 129—6.2 percent—later had an acute attack.7 (That’s pretty high, but this study was conducted in 1993. Some of these patients may not have met the exact criteria for PACS, and we don’t know whether or not the patients had symptoms.) A study conducted in Vellore, India, found that no one in a group of 48 PACS patients went on to have an acute angle closure attack.3
Given the data from these three studies, it’s pretty clear that the risk of acute angle closure is low, although we don’t know exactly how low.
What about patients who get dilated? Pupil dilation makes the angle more crowded, so you’d think that these patients would be much more prone to having an angleclosure attack. However, the data suggests that even in this situation the risk is pretty low. A study conducted in Singapore found that three out of 471 angle-closure suspects (0.64 percent) had an acute attack when dilated.8 A study in Rotterdam found that two out of 149 patients with “narrow angles” (1.3 percent) had an acute attack when dilated.9 (No specific definition of “narrow angles” was provided in the study.) The Baltimore Eye Survey found than none of 38 patients with “occludable angles” had an attack when dilated.10 So even when PACS patients are dilated, the risk is pretty low.
For argument’s sake, let’s assume the risk of acute angle closure attack in a totally asymptomatic patient with PACS is 1 percent. (I think that’s high, but let’s assume it’s correct.) In terms of the number needed to treat, you’d have to do 100 iridotomies to prevent one acute angleclosure attack. As I noted earlier, an LPI is not a totally benign procedure. So the question becomes: Is it worth subjecting the 99 patients who wouldn’t have had an acute angle closure attack to earlier cataract, possible iritis, IOP spikes and dysphotopsias to prevent an acute attack in the 100th patient? That’s really the way to look at it.
My Decision Tree
Eventually I suspect we’ll pin down some biometric parameters that will predict who is at higher risk of getting into trouble. It could be that lens vault or iris thickness will turn out to correlate with acute angle closure attacks. Perhaps it won’t be a static parameter but a dynamic feature we observe with constriction or dilation of the pupil, such as the change in iris volume, that will predict who gets into trouble. (Harry Quigley, MD, has looked at choroidal expansion and shown that this can cause the lens/iris diaphragm to move forward, possibly triggering an acute angle closure attack.) Currently, we don’t have any 21st-century, high-tech provocative test to predict which PACS patients are at risk of developing angle closure. That’s because we don’t even know which biometric parameters are important. This lack of knowledge is why we’re erring on the side of overtreatment, and why current accepted clinical practice involves performing LPIs on every patient with a narrow angle.
So how do I manage patients who qualify as primary angle closure suspects? My approach is to treat the patient, not the angle. Given the lack of evidence-based data to recommend iridotomy, I make the decision on a case-by-case basis. For the vast majority of patients, I choose a course of observation, examining the patient every six to 12 months. History-taking is focused on symptoms that are suggestive of intermittent angle closure. IOP measurement and compression gonioscopy are used to rule out progression from PACS to PAC. Visual fields, OCT and digital disc photography may detect early optic nerve damage and the development of PACG. The development of elevated IOP, PAS or optic nerve damage in a PACS patient indicates the patient is no longer a PAC suspect; at that point intervention is warranted.
However, some circumstances will cause me to proceed with an LPI in an otherwise asymptomatic PACS patient. These include:
• The patient has a history of retinal disease. I’ll perform LPI in a totally asymptomatic PACS patient if the patient is seeing a retinal specialist on a regular basis. Patients with a history of macular degeneration or peripheral retinal tears will likely require dilated exams for the rest of their lives.
• The patient may not return in a timely manner. With some patients, there’s a question as to whether they’re going to return for follow-up visits. They disappear. Those patients can show up five years later with a red, painful eye and a pressure of 60 mmHg.
• The patient will not have timely access to medical care. If a patient is going backpacking in the Sierras for several weeks or volunteers in Africa for several months at a time, he or she may not have prompt access to emergency ophthalmic care if acute angle closure occurs.
• The patient has a family history of acute angle closure, angle closure glaucoma, or blindness resulting from angle closure.
• The patient develops symptoms suggestive of angle closure. In my experience, an acute angle-closure attack doesn’t come out of the blue; there are almost always warning signs. People who come in with acute angle closure have usually had symptoms such as eye pain or headaches in the preceding months, indicating that their intraocular pressure has been going up. Some of these patients have complained to me about migraine headaches, usually on one side (the side with the narrower angle). They’re taking pain or migraine medication. Often LPI results in a complete disappearance of their migraines. However, the complaints are often more vague and ill-defined than the characteristic headache and eye pain we usually associate with elevated IOP.
So, when I encounter a symptomatic patient with PACS, I usually proceed with LPI. In my experience, however, most patients with narrow angles will tell you they haven’t had any symptoms. Those are the patients I observe.
Time to Rethink?
It’s easy to understand why we tend to laser most patients who come in with narrow angles, but no other signs or symptoms. The last thing we want is for any patient to lose vision from an acute angle closure attack that might have been prevented. Nevertheless, the numbers suggest that we may be causing some ocular morbidity in a large number of patients in our attempt to prevent dire consequences in a very small number of patients. It’s worth considering a more tailored approach. REVIEW
Dr. Tanaka is a clinical instructor at California Pacific Medical Center in San Francisco, and in private practice in San Francisco and Oakland. He is a consultant for Ellex and Allergan, and a speaker for Glaukos and Aerie Pharmaceuticals, but he has no financial interests in the subject matter of this article. You can reach him at ghtanakamd@gmail.com.
1. Azuara-Blanco A, Burr J, Ramsay C, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomized controlled trial. Lancet 2016;388:1389-97.
2. Jiang Y, Chang DS, Zhu H. Longitudinal changes of angle configuration in primary angle-closure suspects. Ophthalmol 2014;121:9:1699-1705
3. Thomas R, George R, Parikh R, et al. Five year risk of progression of primary angle closure suspects to primary angle closure: A population based study. Br J Ophthalmol 2003;87:450. 4. Lim LS, Husain R, Gazzard G, Seah HK, Aung T. Cataract progression after prophylactic laser peripheral iridotomy: Potential implications for the prevention of glaucoma blindness. Ophthalmology 2005;112:8:1355-9.
5. Vijaya L, Asokan R, Panday M, George R. Is prophylactic laser peripheral iridotomy for primary angle closure suspects a risk factor for cataract progression? The Chennai Eye Disease Incidence Study. Br J Ophthalmol 2017;101:5:665-670
6. Ye T, Yu Q, Peng S, et al. [Six year follow-up of suspects of primary angle-closure glaucoma]. Zhonghua Yan Ke Za Zhi 1998;34:167.
7. Wilensky JT, Kaufman PL, Frohlichstein D, et al. Followup of angle-closure glaucoma suspects. Am J Ophthalmol 1993;115:338.
8. Lavanya R, Baskaran M, Kumar RS, Wong HT, Chew PT, Foster PJ, Friedman DS, Aung T. Risk of acute angle closure and changes in intraocular pressure after pupillary dilation in Asian subjects with narrow angles. Ophthalmology 2012;119:3:474-80
9. Wolfs RC, Grobbee DE, Hofman A, deJong PT. Risk of acute angle-closure glaucoma after diagnostic mydriasis in nonselected subjects: The Rotterdam Study. Invest Ophthalmol Vis Sci 1997;38:12:2683-7.
10. Patel KH, Javitt JC, Tielsch JM, Street DA, Katz J, Quigley HA, Sommer A. Incidence of acute angle-closure glaucoma after pharmacologic mydriasis. Am J Ophthalmol 1995;120:6:709-17. 11. Zhang X, Liu Y, Wang W, et al. Why does acute primary angle closure happen? Potential risk factors for acute primary angle closure. Surv Ophthalmol 2017t;62:5:635-647.



