Retina
The week kicked off with the muchanticipated interim results from the Comparison of AMD Treatments Trial, which compares Lucentis and Avastin for the treatment of wet AMD. The study, which was presented by Daniel Martin, MD, of the Cleveland Clinic, and Maureen Maguire, PhD, of University of Pennsylvania, compares monthly and p.r.n. treatments of both medications using a non-inferiority paradigm. The protocol defines a noninferiority limit of five letters, and by these criteria none of the possible pairwise comparisons showed inferiority. At 36 months, the mean visual acuity gains for each of the four treatment groups were within 0.6 letters.
One of the striking results of the study was the acuity gain observed in both p.r.n. groups. Previous studies of less-frequent dosing had shown that most of gains in visual acuity were lost when the frequency of administration was reduced.1-3 The major difference between CATT and earlier studies is that the CATT p.r.n. protocol specified retreatment upon evidence of disease activity. In previous studies, a minimum threshold of retinal thickness was set, and patients were retreated only when they exceeded this threshold. CATT patients in p.r.n. treatment groups received a mean of seven (Lucentis) or eight (Avastin) injections during the first year, which is greater than the annual injection numbers in previous studies.
In a related presentation sponsored by Genentech, Emily Gower, PhD, of Johns Hopkins presented results of a retrospective safety study that used Medicare claims data to compare rates of adverse outcomes in patients receiving either Lucentis or Avastin for wet AMD. (Gower E, et al. IOVS 2011;52:ARVO E-Abstract 6644) After adjustment for baseline co-morbidities, patients receiving Avastin were shown to have an 11-percent higher risk of mortality and a 57-percent higher risk of hemorrhagic cerebro-vascular accident relative to patients receiving Lucentis. No significant differences were observed in rates of myocardial infarction or ischemic CVA. Patients receiving Avastin were also 19 percent less likely to develop ocular hypertension or glaucoma, but 80 percent more likely to have ocular inflammation and 11 percent more likely to have cataract surgery. Although these results suggest that the safety of Lucentis is more favorable than that of Avastin, this study was limited by its retrospective nature and the lack of information on potential confounding variables such as smoking.
A special interest group, “Synthesizing the Latest Data from DME and AMD Clinical Trials,” provided a broader view on how the latest clinical Trials for wet AMD and DME (including CATT) may affect future therapies. (Nguyen Q. IOVS 2011;52:ARVO Eabstract 245) Presentations included results from the VIEW-1 and VIEW-2 trials of intravitreal VEGF Trap-Eye (Regeneron, Tarrytown, N.Y.), which was shown to be statistically non-inferior to monthly Lucentis in prevention of moderate vision loss associated with wet AMD. These results suggest that VEGF Trap-Eye may be able to reduce the treatment burden on patients by allowing less frequent intravitreal injections. In a trial sponsored by a research grant from Genentech, patients who had received standard Lucentis dosing for a minimum of 12 months were treated with a fourfold higher “superdose” of the drug (2 mg every four to six weeks for eight months). (Chen E. IOVS 2011;52:ARVO E-Abstract 1653) Results showed gains in acuity and reductions in retinal thickness demonstrating that higher doses may be beneficial to patients who don’t initially respond to anti-VEGF treatment. This study has provided rationale for the Genentech-sponsored HARBOR trial.
Positive interim data from a Phase II trial of the VEGF Trap-Eye for treatment of DME provide further support for anti-VEGF based methods for macular disease beyond AMD. (Tolentino M. IOVS 2011;52:ARVO Eabstract 6646) Based on these results, along with previous studies such as the READ-2, RESTORE and the DRCR.net trials,4-6 anti-VEGF therapy is likely to become the first-line treatment for DME. When asked about whether Lucentis or Avastin was a better treatment option for DME, the panelists emphasized that it wasn’t appropriate to extrapolate from the results of the CATT study and concluded that the two treatments are equally safe and effective. Given the cardiovascular adverse effects associated with systemic anti-VEGF therapy for cancer, the elevated cardiovascular risk of a diabetic patient population and the greater systemic exposure from intravitreal Avastin relative to Lucentis, there are legitimate reasons to be concerned about the safety of Avastin for DME, despite the low systemic exposure after intravitreal administration. Panel members also discussed a future DRCR.net study comparing Avastin and Lucentis for the treatment of DME, as well as recent positive results of the FAME study evaluating the safety and efficacy of the fluocinolone implant (Iluvien; Alimera Sciences, Alpharetta, Ga.) for the treatment of DME. (Antoszyk A. IOVS 2011;52:ARVO E-Abstract 6645) Compared to anti-VEGF therapy, the steroid implant offers greater convenience based upon its two-to-three year duration while anti-VEGF therapy seems to provide greater visual gain with fewer adverse effects.
Geographic atrophy was the subject of another special interest group meeting. (Bird A. IOVS 2011;52:ARVO Eabstract 334) A theme of the session was the multi-factorial nature of the disease and the impediments this places on diagnosis and treatment. Studies described included those documenting loss of photoreceptors outside the edge of the typical GA lesion. This suggests that photoreceptor loss may precede the development of the atrophic lesion, and that the reduced visual function associated with early AMD may be explained primarily by loss of retinal photoreceptors. These studies underscore the need for better diagnostic tools. Jay Ambati, MD, of the University of Kentucky, then described his recent work showing that geographic atrophy is associated with pathologic accumulation of RNA, and that a specific ribonuclease (known by the acronym DICER) is responsible for preventing the RNA accumulation in healthy RPE. Dr. Ambati also showed a reduced expression of DICER in GA eyes, and was able to recapitulate aspects of the GA phenotype in mice by either DICER inhibition or RNA over-expression. This research, which was recently described in the journal Nature,7 also provides a possible explanation for the lesion expansion characteristic of GA: many cells, including RPE cells can internalize long dsRNAs. Dr. Ambati’s work suggests that expansion of GA lesions over time may be due to the internalization of RNA by RPE cells at the growing lesion border.

Glaucoma
This year’s meeting put an emphasis on imaging, which was apparent in the many posters examining new approaches to diagnosis and progression of glaucoma. For example, researchers described the first use of the Heidelberg Retina Tomograph in a placebocontrolled clinical trial. (Hoffman E. IOVS 2011;52:ARVO E-Abstract 205; Stefano M. IOVS 2011;52:ARVO Eabstract 3061) As part of the European Glaucoma Prevention Study, they established that baseline HRT measurements of optic disk topography were highly correlated with other retinal changes, and so may provide an appropriate measure for monitoring progression of the disease. Other refinements of spectral- and time-domain were compared with established methods in other presentations (Elledge J. IOVS 2011;52:ARVO E-Abstract 138; Ristau T. IOVS 2011;52:ARVO E-Abstract 144; Deak GG. IOVS 2011;52:ARVO E-Abstract 153) including several reports from the EGPS.
This year’s meeting put an emphasis on imaging, which was apparent in the many posters examining new approaches to diagnosis and progression of glaucoma. For example, researchers described the first use of the Heidelberg Retina Tomograph in a placebocontrolled clinical trial. (Hoffman E. IOVS 2011;52:ARVO E-Abstract 205; Stefano M. IOVS 2011;52:ARVO Eabstract 3061) As part of the European Glaucoma Prevention Study, they established that baseline HRT measurements of optic disk topography were highly correlated with other retinal changes, and so may provide an appropriate measure for monitoring progression of the disease. Other refinements of spectral- and time-domain were compared with established methods in other presentations (Elledge J. IOVS 2011;52:ARVO E-Abstract 138; Ristau T. IOVS 2011;52:ARVO E-Abstract 144; Deak GG. IOVS 2011;52:ARVO E-Abstract 153) including several reports from the EGPS.
There were several posters outlining clinical results of Rho kinase inhibitors as a new therapy for glaucoma. In a Phase II trial, the Rho kinase inhibitor AR-12286 (Aerie Pharmaceuticals, Bridgewater, N.J.) was compared to latanoprost for efficacy and safety. (Searle J. IOVS 2011;52:ARVO E-Abstract 217) Both AR-12286 and latanoprost lowered IOP, with no clinically significant differences. Another study tested Kowa’s Rho kinase inhibitor K-115 in a study that compared the 24-hour time course of IOP effects of placebo vs. two concentrations (0. 2%, 0.4%). (Yamamoto T. IOVS 2011;52:ARVO E-Abstract 216) The researchers, who are consultants for Kowa, found the higher dose yielded a larger decrease in pressure and both concentrations displayed a similar frequency and severity of adverse effects.
Several studies were aimed at improving the therapeutic profile of prostaglandin analogues. Novagali Pharma presented pre-clinical data comparing traditional aqueous formulations of latanoprost with an emulsion formulation of the company’s drug Catioprost. (Garrigue J. IOVS 2011;52:ARVO Eabstract 238) The data suggest that this formulation change may provide a longer duration of action and an improved safety profile. A similar report showed the efficacy and safety of a gel-based formulation of timolol. (Huguet P. IOVS 2011;52:ARVO E-Abstract 227)
The growing appreciation of commonalities between AMD, glaucoma and Alzheimer’s was a topic for discussion in a special interest group that focused on shared neurodegenerative pathways and therapeutic targets in glaucoma and AD. (Saragovi U. IOVS 2011;52:ARVO E-Abstract 243) For example, the neurotoxic â-amyloid peptide associated with AD has been identified in retinal tissue of glaucomatous rats. Other researchers established that another amyloid-derived peptide, sAPPá, is a potent neuro-protectant Against rotenone-induced RGC loss in human retinal cultures. (Waugh H. IOVS 2011;52:ARVO E-Abstract 3078) They also showed that high levels of sAPPá are present in the vitreous of human eyes, suggesting a natural protective function that may be disrupted as part of the pathology of glaucoma.
Other studies tested drugs that had been developed for neurodegenerative disorders for their ability to protect RGCs in experimental glaucoma. Galantamine, a cholinesterase inhibitor approved for AD, was protective against both retinal and vascular cell damage in a rat model of glaucoma. (Almasieh M. IOVS 2011;52:ARVO Eabstract 2608) This effect was prevented by cholinergic antagonists demonstrating that the drug acts via activation of cholinergic receptors. Adenosine receptors also appear to be possible targets for neuroprotective intervention; the A3 receptor is downregulated in a rat model of ocular hypertension, while the A3-specific agonist Cl-IB-MECA is protective against hypertension-induced RGC apoptosis. (Galvao J, et al. IOVS 2011;52:ARVO E-Abstract 3087)
Anterior Segment Disease
One of the more interesting presentations of the week came from Valeri Shestopalov, PhD, of the University of Miami, and colleagues, who employed molecular techniques to provide a comprehensive assessment of microbiological diversity at the ocular surface. (Shestopalov V, et al. IOVS 2011;52:ARVO E-Abstract 1488) In this study, a more comprehensive assessment of the ocular microbiome was developed using amplified ribosomal RNA sequences combined with sophisticated sequencing technologies. Sequences obtained from healthy ocular surface samples and from contact lenses were compared with those of known genomic databases. This approach identified an average of 221 microbial species per subject, and showed that while there was a high degree of individual variation, a core group of organisms including commensal, environmental, and potentially pathogenic bacteria, fungi and viruses was identified in all samples. A karyotyping analysis (designated with the acronym BRISK) showed another surprising finding: Conjunctival samples yielded 17 core species, while 28 core species were found in contact lens samples.
There were several presentations dealing with use of the antibiotic azithromycin for treatment of corneal diseases beyond infections. Macrolide antibiotics are known to have a variety of anti-inflammatory effects in addition to antimicrobial activity, but the mechanisms are still unclear. A study from the University of North Carolina and Inspire Pharma showed that the drug can inhibit bacterial lipase secretion at concentrations below that used for antibiotic activity. The researchers suggest that this may a provide rationale for use of the drug in blepharitis. (Nandi S, et al. IOVS 2011;52:ARVO E-Abstract 1491) Two company-sponsored animal studies examined the drug’s utility in the treatment of inflammatory conjunctivitis and in a model of dry-eye disease. (Fernandez P, et al. IOVS 2011;52:ARVO E-Abstract 1931; Sandri Z, et al. IOVS 2011;52:ARVO E-Abstract 1124) Using a model of lipopolysaccharide (LPS)-induced rat conjunctivitis, topical azithromycin showed a significant reduction in clinical signs when compared with the vehicle group. Furthermore, expression of pro-inflammatory molecules such as IL-6 and MMP-2 were significantly reduced compared with vehicle-administered animals, suggesting that azithromycin may act by interfering with these inflammatory pathways. Similar results were seen in a mouse model of dry eye, where the drug reduced expression of IL-1â and significantly lowered corneal staining compared to control animals. In a human trial, researchers at the University of Rome presented data from a retrospective study of ocular rosacea suggesting that azithromycin may provide a more rapid relief than systemic doxycycline. (Mantelli F, et al. IOVS 2011;52:ARVO E-Abstract 1500)
Animal studies designed to explore the role of allergic conjunctivitis in corneal graft rejection suggested that rather than altering the immune privilege of corneal allografts, AC appears to alter corneal allograft-induced T cell function, perhaps via the action of AC-induced IL-4. (Reyes N, et al. IOVS 2011;52:ARVO E-Abstract 1141) Another poster demonstrated that in mice, pollens or pollen extracts can stimulate production of pro-inflammatory cytokines and chemokines in conjunctival and corneal epithelium. (Li D. IOVS 2011;52:ARVO E-Abstract 2912)
Several presentations characterized the pre-clinical and clinical properties of the new anti-histamine alcaftadine.
Pre-clinical data provided a link between the drug and the inflammatory nature of chronic allergy by demonstrating effects on both epithelial tight junctions, and on recruitment of inflammatory cells such as eosinophils. (Lane K, et al. IOVS 2011;52:ARVO Eabstract 6417; Gallois-Bernos A, et al. IOVS 2011;52:ARVO E-Abstract 6426) These effects, together with the traditional antagonism of histamine action, are thought to underlie the long duration of alcaftadine action established in the clinical studies according to presentations by investigators affiliated with Johnson & Johnson. (Gomes P, et al. IOVS 2011;52:ARVO E-Abstract 6421; Greiner J, et al. IOVS 2011;52:ARVO E-Abstract 6427)
Interest in corneal inflammation and corneal graft healing was highlighted by several studies examining cyclosporine as a potential anti-inflammatory therapy. Studies with either traditional formulations or polymeric carriers showed that the drug can act as an anti-inflammatory in several models, but that this effect is coupled with a suppression of corneal nerve regeneration. (Chaudhary S, et al. IOVS 2011;52:ARVO Eabstract 2027; Bourges J, et al. IOVS 2011;52:ARVO E-Abstract 3212) This effect was also shown in emulsions, in a study sponsored by Novagali Pharma. (Liang H, et al. IOVS 2011;52:ARVO E-Abstract 2026) A clinical trial employing an emulsion-based formulation of cyclosporine for dry eye reported clinically significant improvement in corneal staining without significant improvement in patient-reported symptomatology. (Lemp M, et al. IOVS 2011;52:ARVO E-Abstract 3821) Most of the study’s investigators are employees of the drug’s maker, Novagali Pharma. This result highlights a long-standing challenge for clinical trials in dry eye: the difficulty in establishing improvements in both signs and symptoms of disease.
There was no shortage of clinical and pre-clinical dry-eye studies, many of which focused on improved clinical methods and metrics for screening and development of potential therapeutics. An enhanced murine model for dry-eye drug screening was described by one group of investigators. (Crawford K, et al. IOVS 2011;52:ARVO E-Abstract 3871) Their method employs a controlled adverse environment combined with subcutaneous scopolamine to reduce tear production. Using this approach, they were able to show increased corneal staining following CAE exposure that was made more severe by scopolamine-treatment. This study also monitored tear cytokines, snd showed increases in IFN-ã, IL-10, IL-13 and TNF-á. Both corneal staining and cytokine levels responded to several positive control medications, confirming that the model accurately reflects results from human CAE trials.
Previous work from our research group at Ora had focused blink rates and blink patterns as one approach toward better dry-eye metrics.8,9 This year, several presentations, from our group and others, explored blink patterns and “blink biology,” and began to apply the lessons learned in the clinic. It’s clear from several studies that the relationship between tear-film breakup and blink rate is complex, and involves sub-populations of patients who respond differently to changes in tear-film stability. (Lafond A, et al. IOVS 2011;52:ARVO E-Abstract 3845; Johnston P, et al. IOVS 2011;52:ARVO E-Abstract 3843) In addition, dry-eye patients show a significant increase in full blinks (eyelids touch) and a corresponding decrease in partial blinks when compared to those with normal tear function. (Contractor M, et al. IOVS 2011;52:ARVO E-Abstract 3830) One adaptive response observed in patients with dry eye is the squeeze blink, in which the upper and lower lids close and exert force on each other. (Rodriguez J, et al. IOVS 2011;52:ARVO E-Abstract 3725) This type of blink causes an initial instability in the tear film, perhaps due to the over-secretion of meibum, followed by several rapid full blinks and a recovery of a uniform, stable film. Based upon their results, the authors point out the need for caution when comparing a natural blink to directed blink activity (such as squeeze blinks) in a diagnostic setting.
In several clinical studies, Alcon/ Novartis investigators and colleagues examined blink patterns in the setting of a dry eye or meibomian gland dysfunction therapy trial. (Griffin J, et al. IOVS 2011;52:ARVO E-Abstract 3847; Contractor M. IOVS 2011;52:ARVO E-Abstract 3830) Systane Balance (Novartis, Fort Worth, Texas) demonstrated the ability to significantly increase tear-film breakup times and reduce blink rate in dry-eye patients. In addition, an analysis of full versus partial blinks showed that the use of Systane Balance normalized the pattern of these blinks in patients with dry eye. While these results are relatively clear cut, blink patterns in patients with MGD were more complex. (Welch D, et al. IOVS 2011;52:ARVO E-Abstract 3813) While the MGD patients, like those with dry eye, display a higher proportion of full vs. partial blinks when compared to normal subjects, they do not show significant differences in either blink rates or in tear-film breakup times.
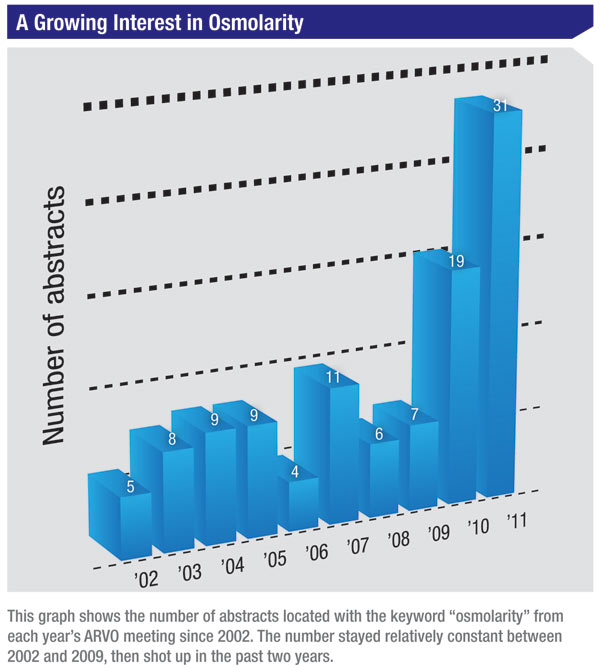 |
Until recently, tear film analysis could not be carried out in clinical practice due in part to the elaborate tear sample collection and extensive laboratory procedures required. The TearLab Osmolarity System allows nanoliter volumes of tear fluid to be measured with the same analytical performance as laboratory osmometers. Since it is assumed that osmolarity values differ between the tear meniscus and the bulbar tear film, one ARVO study examined whether the angle of the TearLab chip toward the tear meniscus during sample collections influenced readings. The authors concluded that tear film osmolarity values varied significantly, depending on exactly where (within the tear meniscus) the tear-film sample was collected. Despite growing use of osmolarity measurements as a metric in dry eye, it seems that current technology is such that it can only serve as an adjunct to established diagnostic techniques such as corneal staining or Schirmer’s tests. (Wunderlich K, et al. IOVS 2011;52:ARVO E-Abstract 3797)
Smartphones
There may have been no single session of the conference that exemplified the future of vision science and medicine better than the workshop on smartphone applications in ophthalmology moderated by Wayne State University’s Vinay Shah, MD. Dr. Shah and his colleagues presented information on the use and utility of Smartphones as tools in clinical, educational, research and personal settings. They estimated that one out of two physicians uses a personal digital assistant or Smartphone, meaning that half of us are still living in the 20th century. The message was clear: The time to get on the bandwagon is now.
The workshop presented a candy store of applications, from high-level programs for tracking clinical trials, database access and management, to mundane applications for managing schedules and contacts. Applications of interest to ophthalmologists include Glaucoma Calc, which can be used to estimate the risk of a patient with ocular hypertension of developing glaucoma; EyeRoute Mobile (Topcon, Oakland, N. J.), which lets users view ophthalmic images on the go by accessing images and reports stored in the EyeRoute Image Management System; and TrialX Clinical Trials (Applied Informatics, New York), which enables doctors and patients to find relevant clinical trials near them and to connect with the trial investigators. Mobile library applications include the Wills Eye Manual and the Eye Handbook (Digital Medicine), both of which provide updated diagnosis and treatment guidelines, drug information and additional tools targeted to those in the visual science or ophthalmology fields.
As always, a week in Ft. Lauderdale provided a chance to see visionary science, from the fundamentals of molecular ophthalmology to the most up-to-date findings of completed and ongoing clinical trials. With this year’s meeting in the books, we can look forward to ARVO 2012, the last time the conference will meet in this familiar locale.
1. Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology 2009;116:9:1731-9.
2. Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, Shams N. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol 2008; 145:2:239-248.
3. Schmidt-Erfurth U, Eldem B, Guymer R, et al., for the EXCITE Study Group. Efficacy and Safety of Monthly versus Quarterly Ranibizumab Treatment in Neovascular Age-related Macular Degeneration: The EXCITE Study. Ophthalmology 2011; 1185:831-9.
4. Nguyen QD, Shah SM, Khwaja AA, et al. Two-Year Outcomes of the Ranibizumab for Edema of the macula in Diabetes (READ-2) Study. Ophthalmology 2010; 117:2146–2151.
5. Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE Study: Ranibizumab Mono-therapy or Combined with Laser versus Laser Mono-therapy for Diabetic Macular Edema. Ophthalmology 2011; 118:615–625.
6. Three-Year Follow-up of a Randomized Trial Comparing Focal/Grid Photocoagulation and Intravitreal Triamcinolone for Diabetic Macular Edema from the Diabetic Retinopathy Clinical Research Network (DRCR.net). Arch Ophthalmol 2009; 127:3:245-251
7. Kaneko H, Dridi S, Tarallo V, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature 2011; 471:325-332.
8. Ousler GW 3rd, Hagberg KW, Schindelar M, Welch D, Abelson MB. The Ocular Protection Index. Cornea 2008; 27:5:509-13.
9. Walker PM, Lane KJ, Ousler GW 3rd, Abelson MB. Diurnal variation of visual function and the signs and symptoms of dry eye. Cornea 2010; 29:6:607-12.



