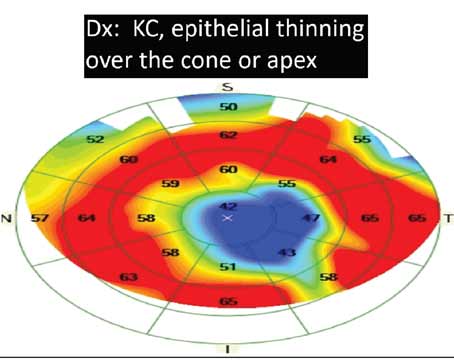
Physicians say that any refractive surgeon worth his salt can perform laser vision correction on the healthy low myope with a thick, normal cornea. It’s the borderline cases—those with a history of radial keratotomy or a past suggestive of herpes simplex, for instance—that really test the surgeon’s mettle. Here, expert refractive surgeons share their thought processes and surgical approaches to three kinds of tough refractive surgery cases.
Herpes Simplex
“A simple, straightforward answer is that when you read the PI booklets for the various excimer lasers, herpes is listed as a contraindication,” says Sonia Yoo, MD, a cornea specialist and refractive surgeon at the University of Miami’s Bascom Palmer Eye Institute. “There are cases, though, of patients who have had a history of keratitis or a remote history of uveitis in whom there’s not a definitive diagnosis of herpes. It’s presumed to be herpes, but there is no viral culture that proved it. So this type of patient may come in with no recurrences of those events and who is on no prophylactic medication, and who really wants refractive surgery. These cases may be candidates for LVC with proper counseling, perhaps even with a perioperative course of oral antivirals to help reduce the risk. Essentially, the risk we’re concerned about is that the excimer, which is UV light, will cause dormant herpes to reactivate, since we know that UV light can cause reactivation.” In this type of patient where it’s not clearly herpes, Dr. Yoo says she’d start an antiviral a week or two prior to the refractive surgery and continue it for several weeks afterward.
Stephen Pascucci, MD, of Bonita Springs, Fla., says that in a patient with herpes, he’d also avoid any topical antiviral agents. “These agents can be, in their own right, toxic to the corneal surface,” he says. “Systemic medications are the way to go.
“Obviously, a good informed consent is important,” Dr. Pascucci adds. “This is so the patient understands that a recurrence during the immediate postop period is a real possibility, but not overly common.”
Dr. Pascucci thinks LASIK might be preferable to PRK in these patients. “I don’t think they have the healthiest epithelium, there’s a relative degree of anesthesia and they probably have a little more dry eye on that side—just probably,” he says. “I wouldn’t want to stress the corneal epithelium and wind up with a non-healing defect, which would certainly complicate the healing and the outcome of the surface ablation. So my vote would be for a LASIK flap in these patients.”
Previous RK
Surgeons say that there are many factors at play in the post-RK patient.
“Typically, these patients are hyperopic with some astigmatism,” says Vancouver, Wash., surgeon Brian Will, who has done many of these cases. “As such, they’re quite debilitated, since they have no good near or distance vision. They’re very frustrated. They have a multifocal cornea as well, and many times their optical zones are pretty small. A surgeon in my area did a lot of RK, including 64-cut RKs with cross-T cuts—pretty much everything you should never do. However, I’ve operated on a few of those and they have done extremely well.”
Dr. Will says the flap creation is key. “There are several things to consider,” he says. “The incisions will tend to separate a bit. Years ago I did some of these cases with the microkeratome and when the pressure went up to 150 mmHg with the suction applied there were times I almost worried that the eye would come apart. But using the femtosecond suction application, the pressure goes up to maybe 70 mmHg, so you can turn on the vacuum and not be concerned that you’re going to have a wound dehiscence. That said, the flap itself is more prone to wound dehiscence as well from the radial incisions. So, in an RK patient, depending on his correction and corneal thickness, I’ll create anywhere from a 130- to 140-µm flap.
“The second thing that needs to be done is make a large-diameter flap,” Dr. Will continues. “So, I’ll typically do at least an 8.8-mm flap, with maybe a 4-percent elliptical oversize. You also have to change your laser’s settings. Your sidewall energy in particular needs to go up at least 10, maybe 15 or even 20 percent. This is because the most difficult part about lifting a LASIK flap is that, when you cut the sidewall in these patients, the old radial incisions don’t tend to cut as well, and that’s when you get in trouble. Also, I use a 90-degree sidewall, vertical straight up and down, which gives a better chance of not getting ingrowth.”
In addition to altering the side-cut energy, surgeons also have to modify their spot-line separation for creating the flap, Dr. Will says. “Typically, we use 5 µm for our spot-line separation,” he says. “But for previous RK patients, I’ll decrease that to 3 µm. So, you’re making your sidewall spot separation and your layer separation closer together—you’re at 3 µm between spots and 3 µm between layers. Again, this is for the purpose of getting a very clean sidewall. For the raster energy, if I’m using a 60-kHz laser, I pretty much always use a double-raster cut. If I’m using the iFS, I may leave the energy the same as in a typical case, or possibly bump it up by 10 percent at most.”
In rare cases, you may need to do an enhancement later. “In these cases, you usually can’t relift the flap easily more than a month postop,” Dr. Will says. “When you try to lift the sidewall, it will be so fused after the previous incisions that you won’t be able to lift it easily and will end up tearing the radial incisions apart. The thing to do on an enhancement is decrease the diameter of the flap by about 0.2 mm and recut the sidewall. So, if your original flap were 8.8 mm wide, you’d use 8.6 mm the second time. As for thickness, if I cut 130 µm on the original, I tend to go back and cut 150 µm on a recut to make sure I transect the old raster bed.”
Rheumatoid Arthritis
Surgeons say patients with RA have eyes that usually don’t respond the same way as the typical refractive surgery patient.
“RA is an auto-immune disease, and patients with auto-immune diseases can have unexpected wound healing issues,” says Dr. Yoo. “So, as a broad statement, I’d say RA patients aren’t good candidates for LVC. Also, if they are on antimetabolites for their disease, they aren’t ideal candidates for LVC because the medication can alter the wound healing response.
“That being said, there might be special circumstances where patients may have a compelling reason for having LVC,” Dr. Yoo adds. “For instance, a patient who is anisometropic and can’t tolerate spectacles because of the difference between the eyes and can’t wear contact lenses because his rheumatoid disease gives him difficulty in handling the contact lenses could be a candidate. If the RA is quiescent and doesn’t require medication for control, that might be a patient in whom you say, ‘OK, with your circumstances, let’s see if you can handle LVC.’ Dry eye would be my number-one concern in these patients, so I’d do a careful tear-film assessment: tear-film breakup time; Schirmer’s to see if she’s producing tears adequately; and fluorescein staining to look for any punctate keratopathy. If there are issues, or he’s just subjectively symptomatic, I treat aggressively with artificial tears and potentially with punctal occlusion and topical cyclosporine-a. I also make sure he doesn’t have any exposure that can be contributing to the dry eye.”
Dr. Pascucci also won’t operate on an RA patient whose disease isn’t well-controlled. But for the ones on whom he will perform LVC, he takes an aggressive dry-eye treatment approach. “In non-RA patients, I tend to very generously lubricate the cornea with non-preserved artificial tears following LASIK, and sometimes add gels at bedtime,” he avers. “I then step up therapy if it appears to be necessary, possibly going to plugs next and then maybe to Restasis after that. In an RA patient, though, I might pull out all the stops. I would plan on plugging him at the conclusion of the procedure and talk to him about the potential benefits of Restasis. If I felt Restasis was warranted, I might start it a week preop to see if we could somehow minimize the dry eye that develops. This type of patient—dry-eye wise—needs a full-court press from the get-go.”
REVIEW