Treating glaucoma with topical drops is problematic. Patient adherence is impossible to guarantee; drops can cause long-term damage to the corneal surface and/or meibomian glands;1,2 and they can wash off the eye, thus requiring a significant amount of drug to be used. (One study found that corneal bioavailability of topical medication was less than 5 percent of the delivered amount.3) Sustained drug delivery could potentially bypass some or all of these problems.
Many different approaches to sustained delivery of glaucoma medications have been investigated. Non-invasive approaches have included drug-impregnated contact lenses, ocular rings that can be inserted under the upper and lower eyelids, collagen shields and drug-eluting punctal plugs. Invasive options have included subconjunctival injections of solutions and drug-eluting polymer microparticles, as well as drug-eluting implants like Allergan’s Durysta, the only implant approved by the FDA at the time of this writing. (Intracameral implants seem to be particularly promising; in addition to Durysta, implants in the pipeline include Travoprost XR, a.k.a. ENV515 [Aerie/Alcon], the iDose TR travoprost implant [Glaukos], and the OTX-TIC travoprost intracameral implant [Ocular Therapeutix].) Other alternatives being investigated include supraciliary implants, microneedles and intravitreal nanosponges.
Overcoming Obstacles
Many of these sustained-drug-delivery systems have been shown to be effective, but practical problems have caused them to become stalled or abandoned. For example, drug-eluting punctal plugs have a number of potential problems to surmount. “In principle, drug-eluting punctal plugs are a good idea,” says Malik Kahook, MD, the Slater Family Endowed Chair in Ophthalmology, chief of the Glaucoma Service and vice chair of translational research at the University of Colorado School of Medicine in Aurora, Colorado. “However, they often fall out post-insertion, and data have suggested that they may not deliver the drug consistently over a 90-day period.”
“A punctal plug is a foreign object with the potential to cause irritation, and rubbing can result in its dislodgement,” notes Parul Ichhpujani, MD, a professor in the Department of Ophthalmology at the Government Medical College & Hospital in Chandigarh, India. “Movement of the plug can affect drug release, because the change in local milieu can cause it to malfunction. Sometimes the patient won’t realize that the plug has dislodged, which would be detrimental to the IOP profile. Other potential problems include that, over a period of time, bacterial buildup may happen, so a preservative will be needed. How will the rate of drug release be titrated in such a scenario? And, there’s no guarantee that the drug release and patient response will be uniform with subsequent plugs.”
Nevertheless, work on this possibility continues. “In 2021, Mati Therapeutics purchased the rights related to the Evolute Punctal Plug Delivery System (PPDS) from Novelion Therapeutics,” notes Dr. Ichhpujani. “Mati has completed multiple Phase II clinical trials using the Evolute platform in glaucoma and ocular hypertension patients. The punctal plug design has demonstrated good lower punctum retention rates of 92 percent and 96 percent in two separate multicenter U.S. clinical trials over a 12-week follow-up period.”
Surgical implants and injections have shown particular promise, but these alternatives also have issues to overcome. Because they’re invasive, they’re potentially associated with risks such as migration of the implant, endophthalmitis, endothelial cell loss and reactions to components of the devices. In addition, not every eye is eligible for such an implant. Finally, if problems arise, these options may not be easy to reverse.
“These approaches provide a few months of drug delivery,” Dr. Kahook says. “However, they carry the risk of injuring the corneal endothelium. They’re not anchored, so they can float around and mechanically injure tissue. Durysta, appears to work well, but it’s only approved for one-time delivery because of the corneal risks.”
Another option that seems to have stalled is sustained delivery via contact lens. Felipe A. Medeiros, MD, PhD, Distinguished Professor of Ophthalmology and vice chair for technology at Duke University in Durham, North Carolina, points out that one of the problems with this approach is that most glaucoma patients are older individuals. “These people are usually in their 60s or 70s, and they’re not usually wearing contact lenses anymore,” he says. “Contact lenses can be hard for some elderly patients to put on the eye. Also, contact lenses can cause serious complications, so you could be exchanging the side effects caused by topical drops for side effects caused by mishandling of the contact lenses, patients sleeping in their lenses, or contact lens-related infections. It’s an interesting approach because it’s noninvasive, but it hasn’t really taken off.”
Here, we’ll review a few of the most promising sustained-release options under investigation, and provide an update on Durysta.
 |
| Allergan’s Durysta, currently FDA-approved for a single use, is now in Phase IV clinical trials to determine whether endothelial cell loss can be minimized with a longer interval between additional applications. |
Allergan’s Durysta
This implant, approved in 2020, is a rod-shaped, biodegradable polymer matrix containing 10 µm of bimatoprost that’s released steadily inside the eye over a period of several months. It can be inserted into the iridocorneal angle in the clinic or operating room, where it dissolves gradually, eliminating the need for removal. Although it’s only designed to last three to four months, studies have found that in some patients the treatment effect lasts far longer.4-10 It has a favorable safety profile, with limited short-term adverse events that tend to be associated with the implantation procedure rather than the implant itself.
Two Phase III studies (ARTEMIS 1 and 2) have supported the safety and efficacy of the implant. Both studies randomized one eye of patients with ocular hypertension or POAG to an intracameral 10- or 15-µg bimatoprost implant, with the other eye receiving topical timolol 0.5% b.i.d. Multiple successive implants were permitted, as needed. Although significant implant biodegradation was observed in the majority of patients by 12 months, residual implants remained visible in the iridocorneal angle in more than 80 percent of the patients at month 20. (Corneal adverse events were more frequent with the larger 15-µg implant due to the volume of implant material in the iridocorneal angle after multiple implant administrations.) Some patients continued to have controlled IOP and stable visual fields more than three years after receiving their last implant.
“The Phase III studies involved three applications of the implant, spaced by four months,” notes Dr. Medeiros, lead investigator in the clinical trials. “This was the original trial design, because in the early experimental work done in dogs, the drug would be gone by four months. In the Phase III trials we found that Durysta lowered the intraocular pressure very well, but in addition to that we were surprised to find that about 80 percent of the patients didn’t need any rescue medication for up to one year after the third implant.
“A portion of the patients in the Phase III trial had significant endothelial cell loss,” he continues. “However, this was mostly related to the presence of stacked implants in the anterior chamber. As a result, Durysta was approved for a single application, which may somewhat limit its clinical applicability.”
“One-time delivery for a system that delivers drug for a few short months isn’t ideal, given that glaucoma is a chronic disease requiring lifetime therapy,” agrees Dr. Kahook. “However, even with limited approval it has a role in patients who need to buy time before having more definitive surgery, and in some other niche areas of treatment.”
Dr. Medeiros points out that since the duration of effect is longer than four months for most patients, the interval between applications in the Phase III trials was likely too short. “In fact,” he says, “our analysis of corneal endothelial cells after a single application doesn’t show significant endothelial cell losses.10 And, a recent study by Robert Weinreb, MD, et al showed that the implant is gone or has minimal size in about 80 percent of eyes after one year.9
“Right now, Phase IV studies are being done,” he adds. “These studies are investigating optimal intervals between applications. With applications spaced out further, the likelihood of endothelial cell loss should be much lower. We hope this will lead to label expansion and approval for repeated applications.”
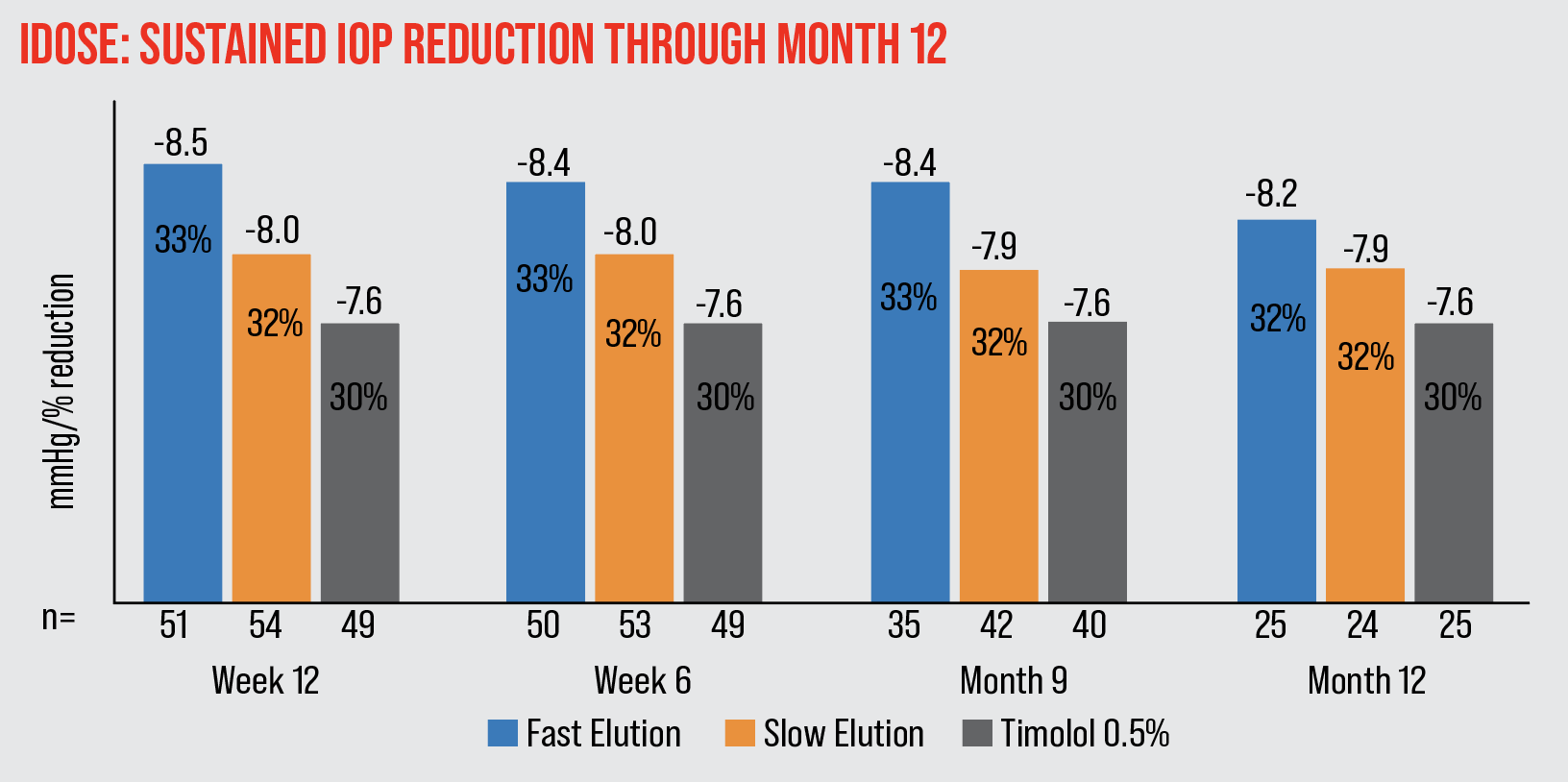 |
| The iDose achieved 7.9-8.5 mmHg (32-33 percent) mean IOP reduction through month 12. |
The iDose Intracameral Implant
Glaukos’ iDose is a 1.8 x 0.5 mm titanium implant designed to provide a steady release of travoprost into the anterior chamber over time. A scleral anchor holds the device in place in the trabecular meshwork. The targeted duration of therapy is six to 12 months. Unlike Durysta, the iDose implant is intended to be removed and replaced with a new device once the drug has been completely released.
A Phase II study found that at the primary 12-week endpoint, patients who had received a fast-eluting implant had a 33-percent decrease in IOP; patients who received a slow-eluting implant had a 32-percent decrease; and a control group receiving timolol twice a day had a 30-percent IOP decrease. The iDose IOP reduction was stable and sustained at 12 months after implantation. There were no serious adverse events.11
In September, Glaukos announced results from two Phase III clinical trials of the iDose. They reported that the system achieved its primary efficacy endpoints at three months in both trials, with high tolerability and a favorable safety profile. Specifically:
- Both the fast-release and slow-release devices were non-inferior to twice-daily topical timolol at three months.
- Ninety-three percent of slow-release iDose patients remained well-controlled on the same or fewer IOP-lowering topical medications at 12 months, compared to 67 percent of the timolol control subjects in both trials.
- Eighty-one percent of patients receiving the slow-release iDose were using no IOP-lowering topical medications at 12 months.
- In terms of tolerability, 98 percent of slow-release iDose subjects continued in the trial at 12 months, versus 95 percent of timolol control subjects.
- In terms of safety, the most frequent adverse event for slow-release iDose subjects was mild transient iritis, at a rate of 6 percent in both trials; the slow-release group also had a conjunctival hyperemia rate of 3 percent. No subjects experienced serious corneal adverse events, including endothelial cell loss, and no periorbital fat atrophy was observed.
“The good thing about iDose TR is that it’s been designed to be removed and replaced with a new iDose TR when the effect wears off,” says Dr. Ichhpujani. “Thus, it potentially offers a continuous, drop-free experience.”
“iDose is the new kid on the block,” notes Dr. Kahook. “The main advantage is that it can deliver the drug for a year or longer—and it seems to work in a manner similar to topical timolol. The possible cons are that you need to have the skill set to do intraoperative gonioscopy for trabecular meshwork implantation, and that the device is on the larger side, with the potential to injure both the cornea and iris if not implanted as indicated by Glaukos.”
Dr. Medeiros agrees. “The iDose requires surgical insertion in the operating room,” he says. “Although the device can provide sustained drug delivery for at least one year, it still needs to be replaced periodically, which may limit the patient population that would benefit from it. For example, patients who would be willing to have repeated surgical interventions over time would most likely be those with more advanced stages of glaucoma. However, those patients are usually already on multiple medications. That means that unless the device can be made to deliver multiple medications at the same time, these patients would still require eye drops.
“Also, this device is designed to be placed in the trabecular meshwork, which requires more skill than a simple injection into the anterior chamber,” he adds. :There may also be a greater risk for complications, although we don’t know that yet. We’ll have to look at the data.”
OTX-TIC Intracameral Implant
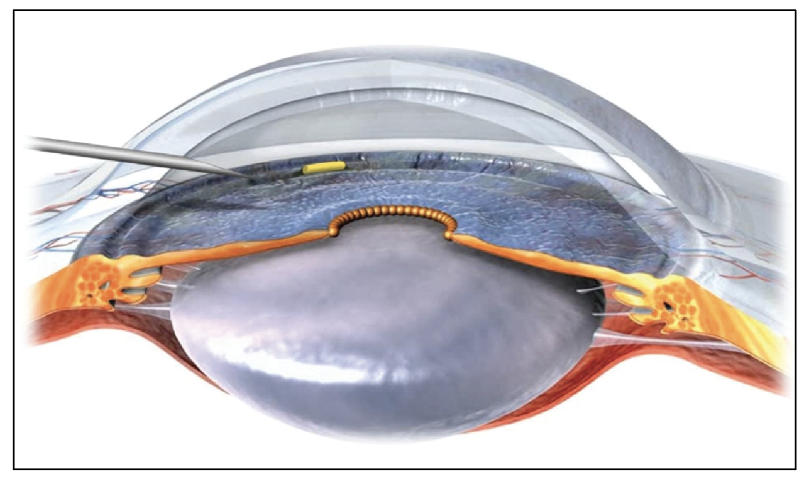 |
| The OTX-TIC implant in the anterior chamber. |
This device, from Ocular Therapeutix, is a proprietary, preservative-free, soft hydrogel platform embedded with travoprost-loaded microparticles that has a low potential for inflammation. According to the company, it has a meshwork that holds the microparticles; after being administered, the meshwork hydrates, allowing the microparticles to dissolve and diffuse the drug. It’s placed into the iridocorneal angle using a 27- or 26-gauge needle, and it’s fully biodegradable. The company says it remains visible during drug diffusion to allow monitoring, but is fully absorbed once the drug has been delivered. The system can be modified to deliver a variety of molecules of different sizes, and can be formulated to provide sustained release for days or months.
According to the company, early clinical trials of the travoprost implant have demonstrated that the system has an acceptable safety profile, maintains a steady level of the drug in the aqueous humor, and produces sustained IOP lowering.12
Travoprost XR
This biodegradable, rod-shaped intracameral implant, placed in the iridocorneal angle of the anterior chamber, uses nanoparticles to provide a steady supply of travoprost inside the eye for six to 12 months. Originally created by Envisia Therapeutics, the system is now being developed by Aerie/Alcon.
Several studies have confirmed its efficacy. A preclinical study using dogs demonstrated a 35.5-percent IOP reduction over an eight-month period.13 A later Phase IIa study involving 21 glaucoma patients compared the implant to topical Travatan Z in the fellow eye. In this study, diurnal IOP dropped by 6.7 mmHg in the study group by day 25 (6.6 mmHg in the topical drop group), thus achieving its primary efficacy endpoint.14 A third, 12-month study involved eyes of open-angle glaucoma patients previously treated with prostaglandins, using topical timolol once daily in the fellow eye for comparison. Mean IOP reduction at 11 months in the study eyes was 6.7 ±3.7 mmHg, or 25 percent—non-inferior to timolol.15
Latanoprost FA SR
This biodegradable, rod-shaped intracameral implant, under development by PolyActiva (Parkville VIC, Australia) will deliver latanoprost. It’s currently in Phase II studies.
IOL-haptic-based Drug Delivery
 |
| The SpyGlass system attaches drug-eluting pads to the haptics of an IOL. It may deliver a drug for three years. |
A new option under development is also worth noting. SpyGlass Pharma (Aliso Viejo, California) is developing a single-piece, hydrophobic acrylic IOL with two small drug-eluting pads that slide onto the haptics, attaching to the IOL at the haptic-optic junction. Once the pads are in place, the IOL and pads can be loaded into a standard IOL injector and placed into the capsular bag through a sub-2.4-mm incision using standard cataract surgical technique. The company explains that the pads remain outside of the visual axis and can continuously elute bimatoprost into the aqueous humor for three years. Preclinical testing in animals has found significant IOP lowering, no detectable systemic exposure and no drug-related adverse events, even with 10 times the standard dose.
An FIH feasibility study conducted outside the United States evaluated safety and efficacy in 23 patients with ocular hypertension or open-angle glaucoma. At three months, findings included:
- a 45-percent mean IOP reduction, regardless of dosage;
- 100 percent of patients were at 18 mmHg or less;
- all patients were off of topical drop therapy;
- no significant adverse events were reported;
- visual outcomes were similar to those achieved with commercially available IOLs.
The company plans to file an Investigational New Drug application within the next six months, and hopes to begin enrollment of patients in a Phase I/II clinical trial in 2023. Dr. Kahook, who founded the company, says that more clinical trial data will be forthcoming soon. “The pipeline for SpyGlass also includes no-drop cataract surgery options, as well as approaches designed to treat uveitis and macular degeneration,” he says.
 |
Looking Ahead
“It’s imperative that we make sure these novel modalities can reach the masses, and not just be restricted to limited ‘boutique’ use,” notes Dr. Ichhpujani. “They have to be safe, effective and easy to use, for a seamless integration into our individual practices. At the moment, there’s not enough econometric evidence to support their use in health-care systems funded by national health care insurance systems, or in countries with primarily out-of-pocket patient expenditure.”
Asked what improvements she’d like to see in the future, Dr. Ichhpujani mentions two things. “Most novel drug delivery systems are focusing on delivering a single drug,” she points out. “The next logical step would be to have a fixed-dose combination drug in a sustained device. Another desirable development would be a novel sustained delivery system coupled with an IOP-monitoring device, such as a drug-impregnated contact lens system.”
“Out of all of the sustained delivery approaches under development, I think those that you inject into the anterior chamber have so far shown the best results in terms of convenience, pressure-lowering efficacy and safety,” says Dr. Medeiros. “Many of the companies developing implants are focused on how the matrix holding the drug biodegrades over time, which is important; as noted earlier, the side effect of corneal endothelial cell loss seems to occur mainly as a result of residual implant material staying in the eye longer than would be desirable.
“I believe further developments in this area will lead to better implants and reduction in potential side effects,” he concludes. “I think the field will be advancing quickly.”
Dr. Medeiros has consulted for Allergan, Aerie, Novartis, Polyactiva and Ocular Therapeutix. Dr. Kahook receives patent royalties from New World Medical and Alcon and is the founder of SpyGlass Pharma. Dr. Ichhpujani reports no relevant financial ties.
1. Perez-Bartolome F, Martinez-de-la-Casa JM, Arriola-Villalobos, et al. Ocular surface disease in patients under topical treatment for glaucoma. Eur J Ophthalmol 2017;27:6:694-704.
2. Uzunosmanoglu E, Mocan MC, Kocabeyoglu S, et al. Meibomian gland dysfunction in patients receiving long-term glaucoma medications. Cornea 2016;35:8:1112-1116.
3. Gause S, Hsu HH, Shafor C, et al. Mechanistic modeling of ophthalmic drug delivery to the anterior chamber by eye drops and contact lenses. Adv Colloid Interface Sci 2016;233:139-154.
4. Shirley M. Bimatoprost implant: First approval. Drugs Aging 2020;37:6:457–462.
5. Craven ER, Walters T, Christie WC, et al. 24-month phase I/II clinical trial of bimatoprost sustained-release implant (Bimatoprost SR) in glaucoma patients. Drugs 2020;80:2:167–179.
6. Lewis RA, Christie WC, Day DG, et al. Bimatoprost sustained-release implants for glaucoma therapy: 6-month results from a phase I/II clinical trial. Am J Ophthalmol 2017;175:137–147.
7. Medeiros FA, Walters TR, Kolko M, et al. Phase III, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology 2020;127:12:1627–1641.
8. Bacharach J, Tatham A, Ferguson G, et al. Phase 3, randomized, 20-month study of the efficacy and safety of bimatoprost implant in patients with open-angle glaucoma and ocular hypertension (ARTEMIS 2). Drugs 2021;81:17:2017-2033.
9. Weinreb RN, Bacharach J, Brubaker JW, et al. Bimatoprost implant biodegradation in the phase 3, randomized, 20-month ARTEMIS studies. J Ocul Pharmacol Ther 2022 Nov 15. doi: 10.1089/jop.2022.0137. [Epub ahead of print]
10. Medeiros FA, Sheybani A, Shah MM, et al. Single administration of intracameral bimatoprost implant 10 microg in patients with open-angle glaucoma or ocular hypertension. Ophthalmol Ther 2022;11:4:1517-1537.
11. Ibach M. Interim results of a prospective phase II study of travoprost intraocular implants. Paper presented at: the American Academy of Optometry Annual Meeting; November 9, 2018; San Antonio, Texas.
12. Walters TR, et al. Presented at the ASCRS Annual Meeting; Boston, MA; May 16, 2020.
13. Navrátil T, García A, Tully J, Maynor B, Ahmed I, Budenz D, Lewis R, Mansberger S, Gilger B, Yerxa B. Preclinical evaluation of ENV515 (travoprost) intracameral implant-clinical candidate for treatment of glaucoma targeting six-month duration of action. Invest Ophthalmol Vis Sci 2014;55:3548–3548.
14. NCT02371746, Safety and Efficacy of ENV515 Travoprost Extended Release (XR) in Patients With Bilateral Ocular Hypertension or Primary Open Angle Glaucoma, https://Clinicaltrials.Gov/Show/ NCT02371746. 2015.
15. Mansberger SL, Conley J, Verhoeven RS, et al. Interim analysis of low dose ENV515 Travoprost XR with 11-month duration followed by dose escalation and 28 day efficacy evaluation of high-dose ENV515. Invest Ophthalmol Vis Sci 2017;58:2110.
16. Brandt JD, Sall K, DuBiner H, et al. Six-month intraocular pressure reduction with a topical bimatoprost ocular insert: results of a phase II randomized controlled study. Ophthalmology. 2016;123:8:1685–1694.
17. Brandt JD, DuBiner HB, Benza R, et al. Long-term safety and efficacy of a sustained-release bimatoprost ocular ring. Ophthalmology. 2017;124:10:1565–1566.
18. Chen MY, Sall KN, Tepedino M, et al. Patient-reported outcomes of bimatoprost ocular ring in an open-label extension study in patients with open-angle glaucoma or ocular hypertension, Invest Ophthalmol Vis Sci 2018;59:1231.
19. Leahy CD, Ellis EJ, Ellis JY, Crawford KS. Efficacy of a topical ocular drug delivery device (TODDD) for the treatment of glaucoma by telemetric measurement of IOP in the normal rabbit. Invest Ophthalmol Vis Sci 2007;48:5816.
20. Crawford K, Ellis J, Rulander J, et al. Sustained delivery of prostaglandin from drug-containing depots using ocular rings in beagles. Invest Ophthalmol Vis Sci 2013;54:5073.
21. Leahy CD, Gutner R, Varney W, et al. Continuous wear non-invasive device for sustained ocular drug delivery. Invest Ophthalmol Vis Sci 2014;55:13:481.